Bot. Bull. Acad. Sin. (2003) 44: 257-265
Lee et al. — Regulation of sucrose phosphate synthase of sweet potato callus
Regulation of sucrose phosphate synthase of the sweet potato callus is related to illumination and osmotic stress
Mu-Ho Lee1, Chien-Chi Yang1, Heng-Long Wang2, and Ping-Du Lee1,*
1Graduate Institute of Agricultural Chemistry, National Taiwan University, Taipei, Taiwan 106
2Department of Life Science, National University of Kaoshiung, Kaohsiung University Rd., Kaohsiung, Taiwan 811
(Received January 27, 2003; Accepted June 6, 2003)
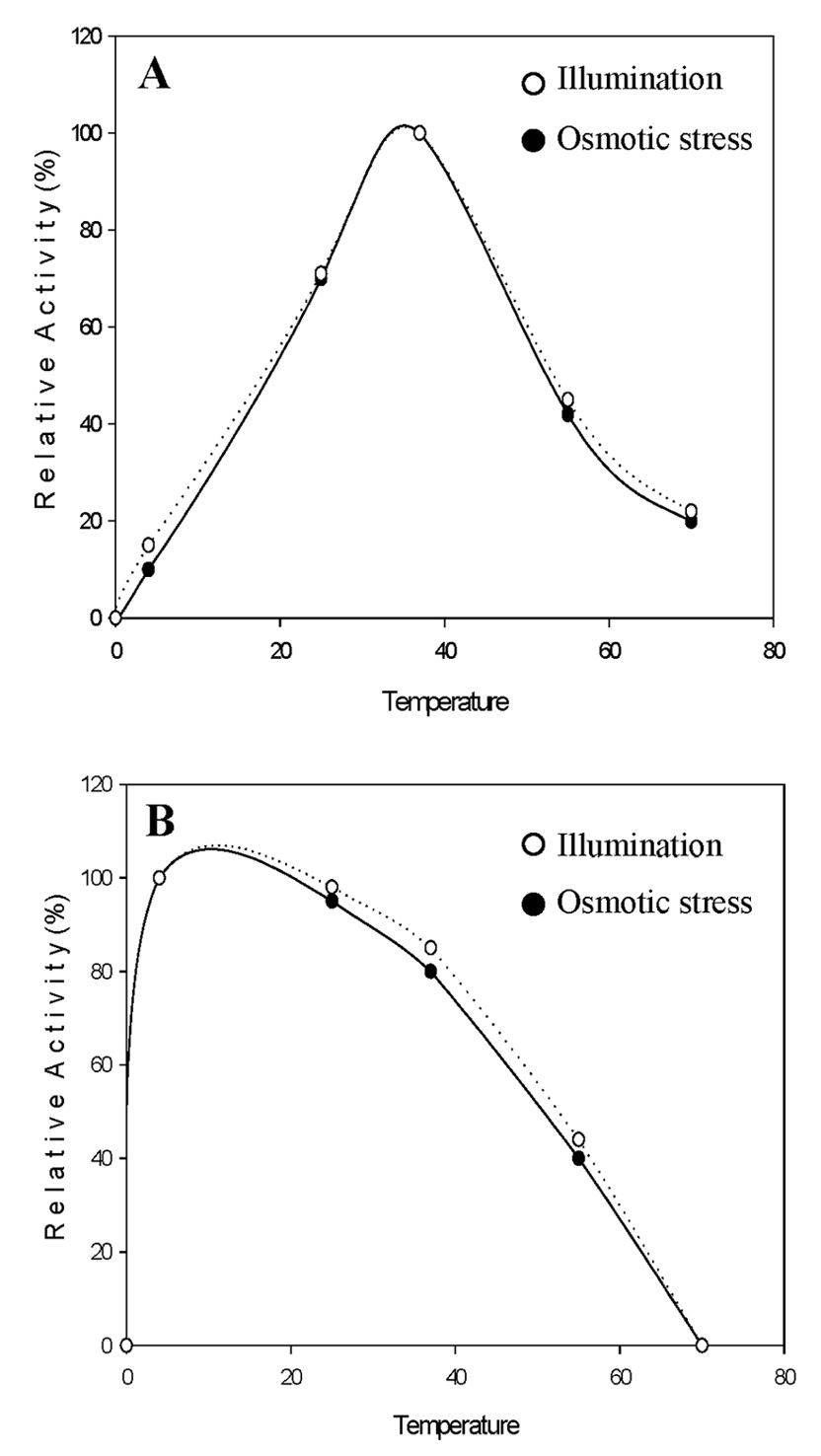
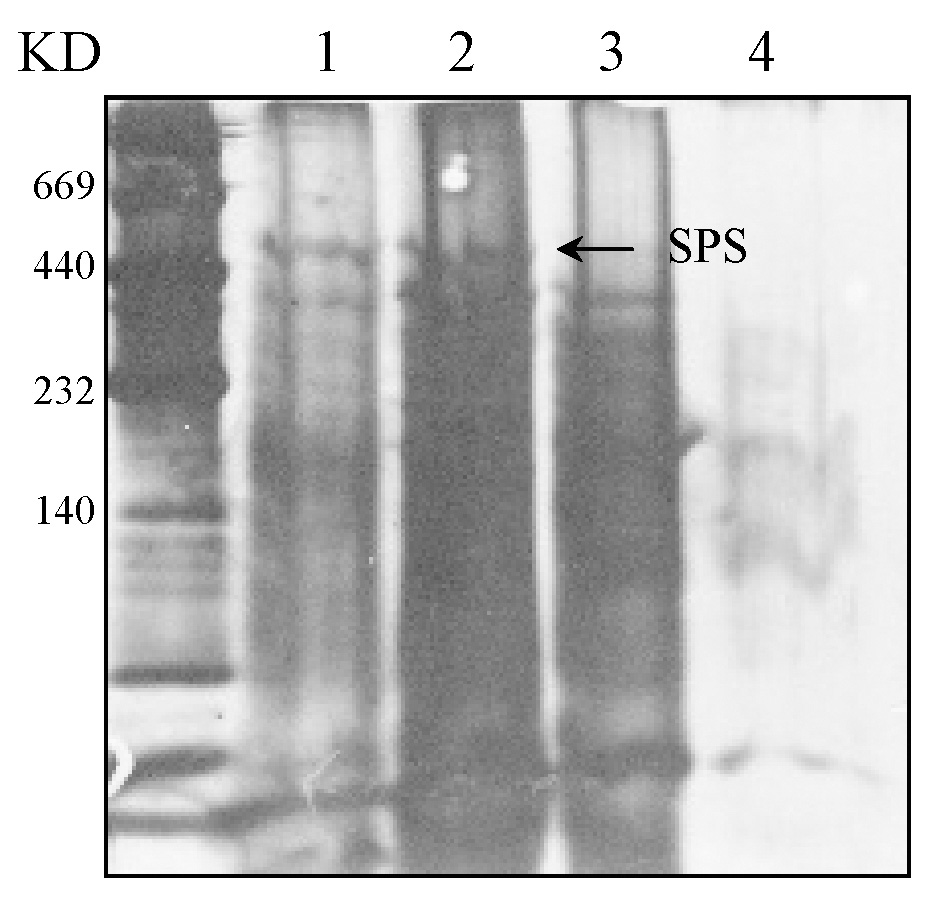
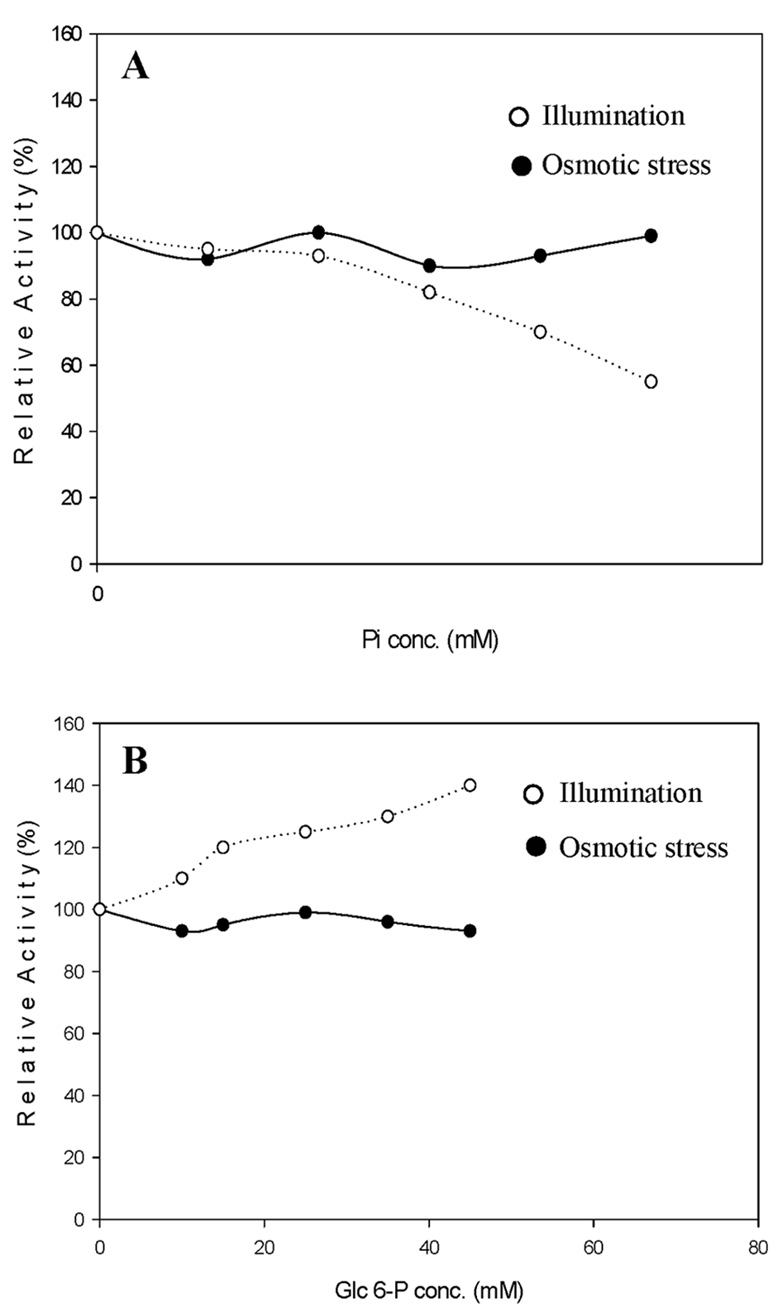
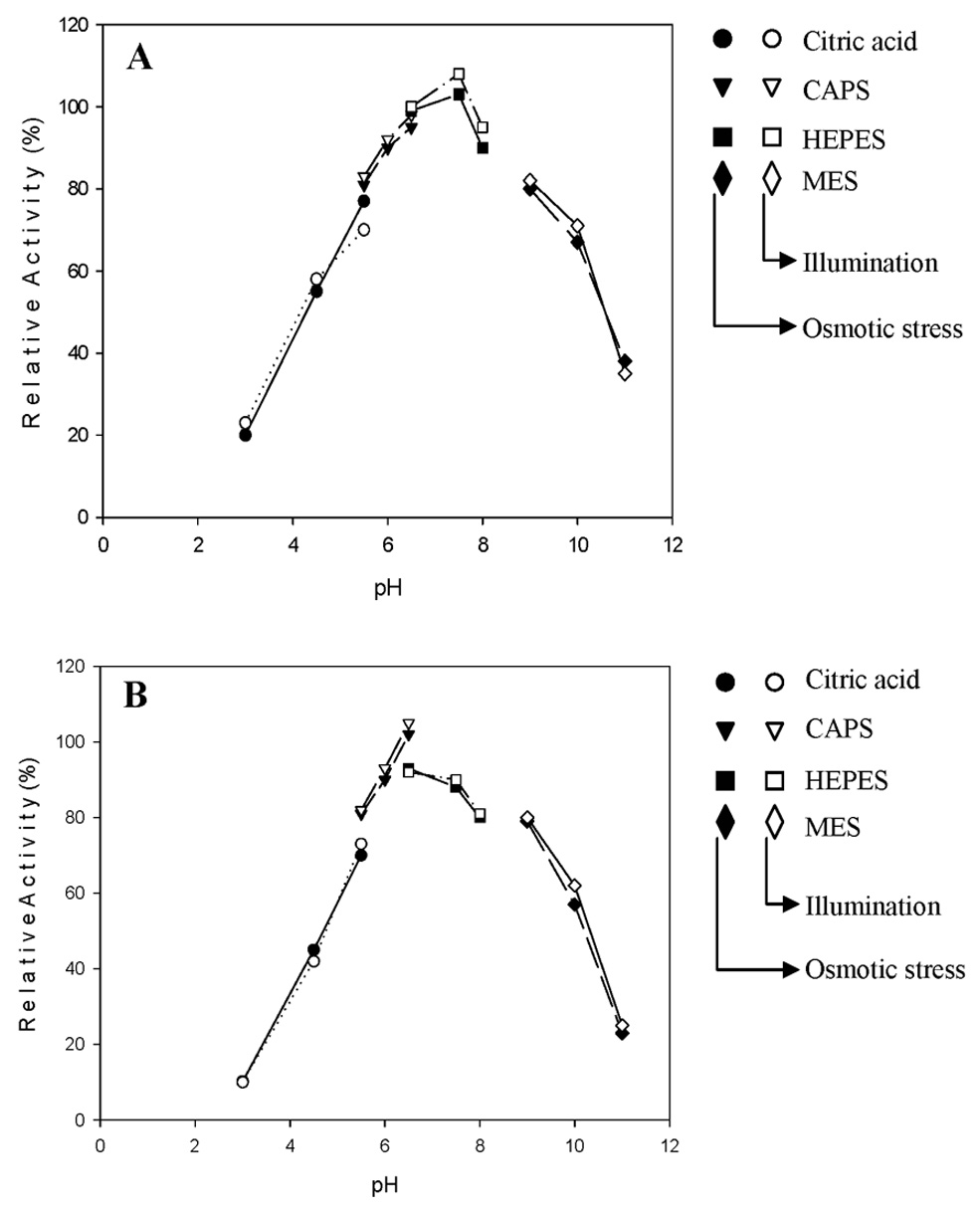
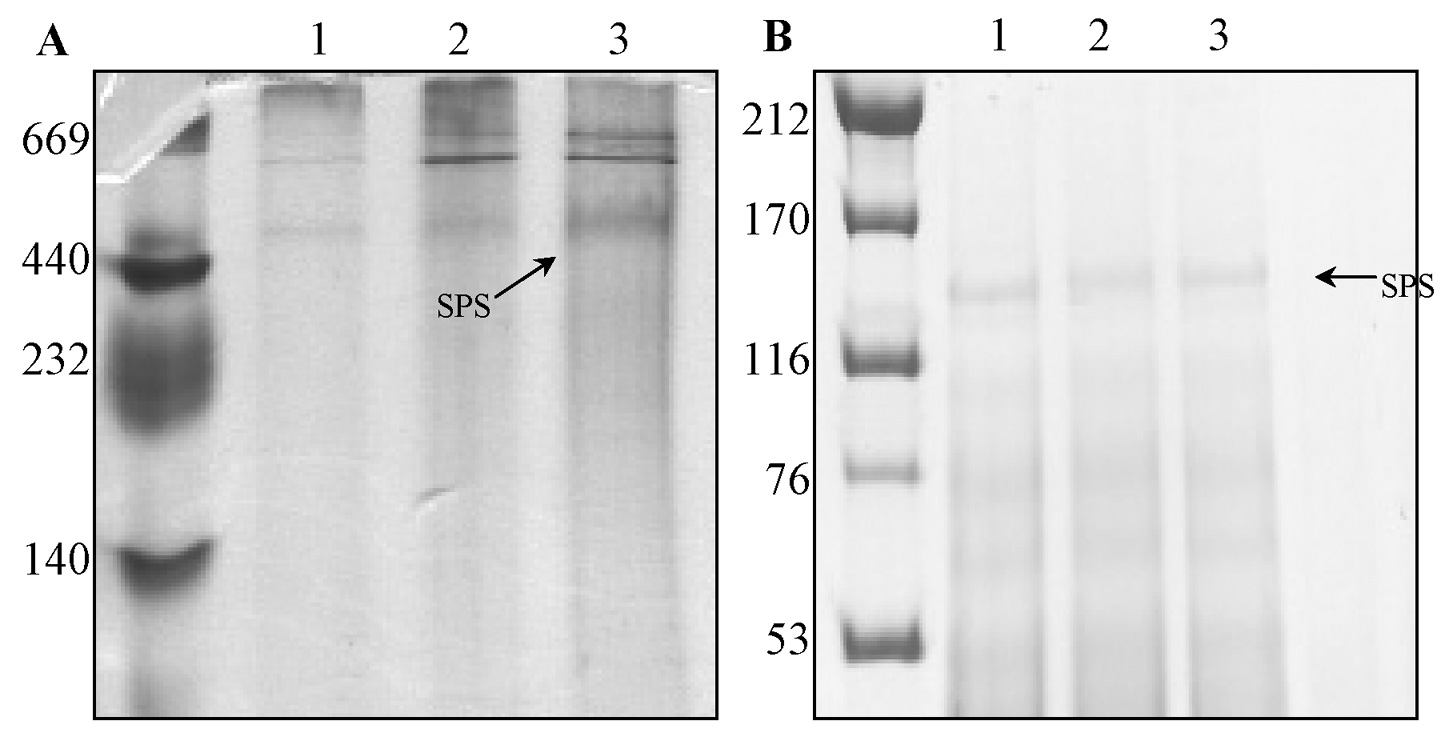
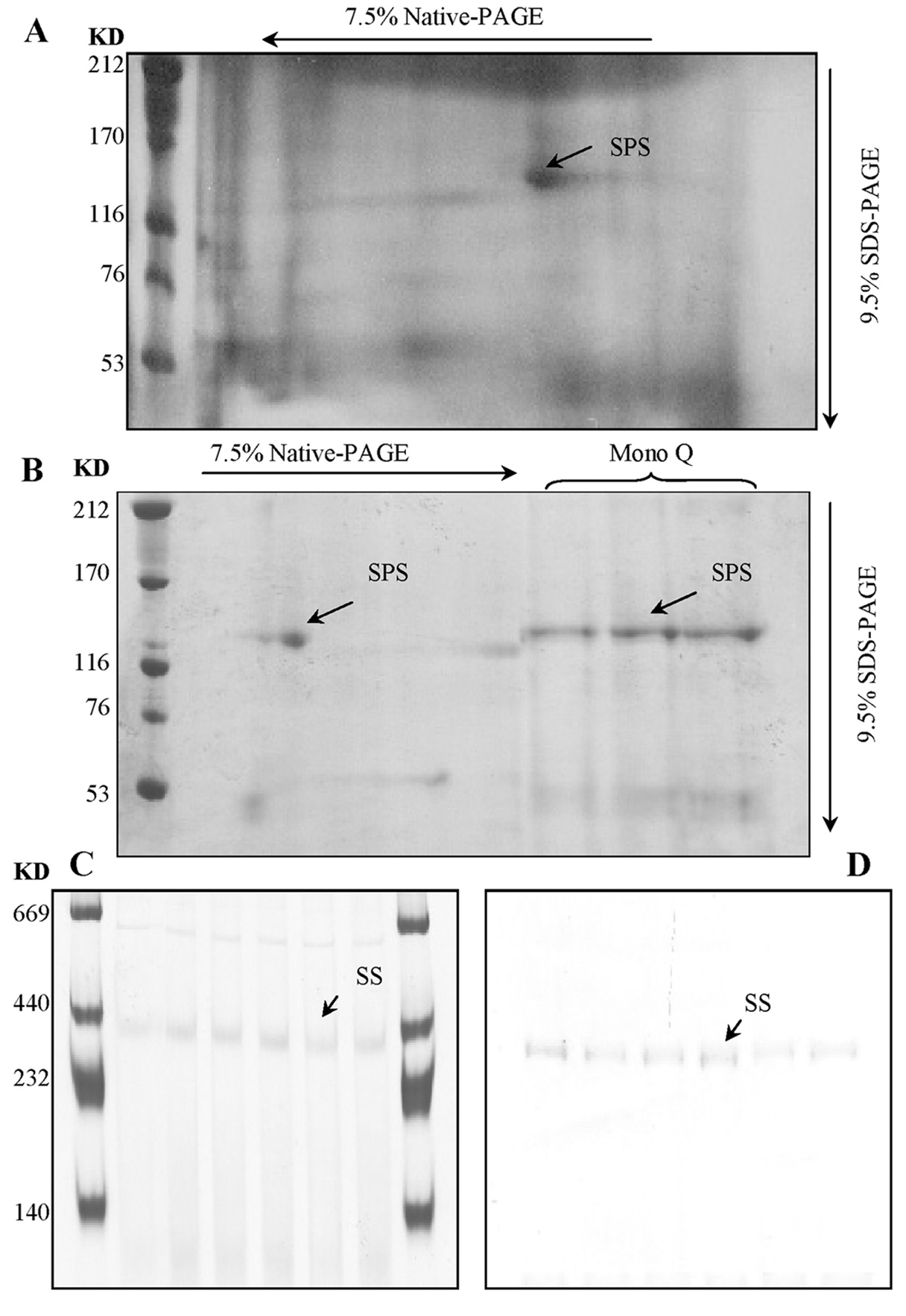
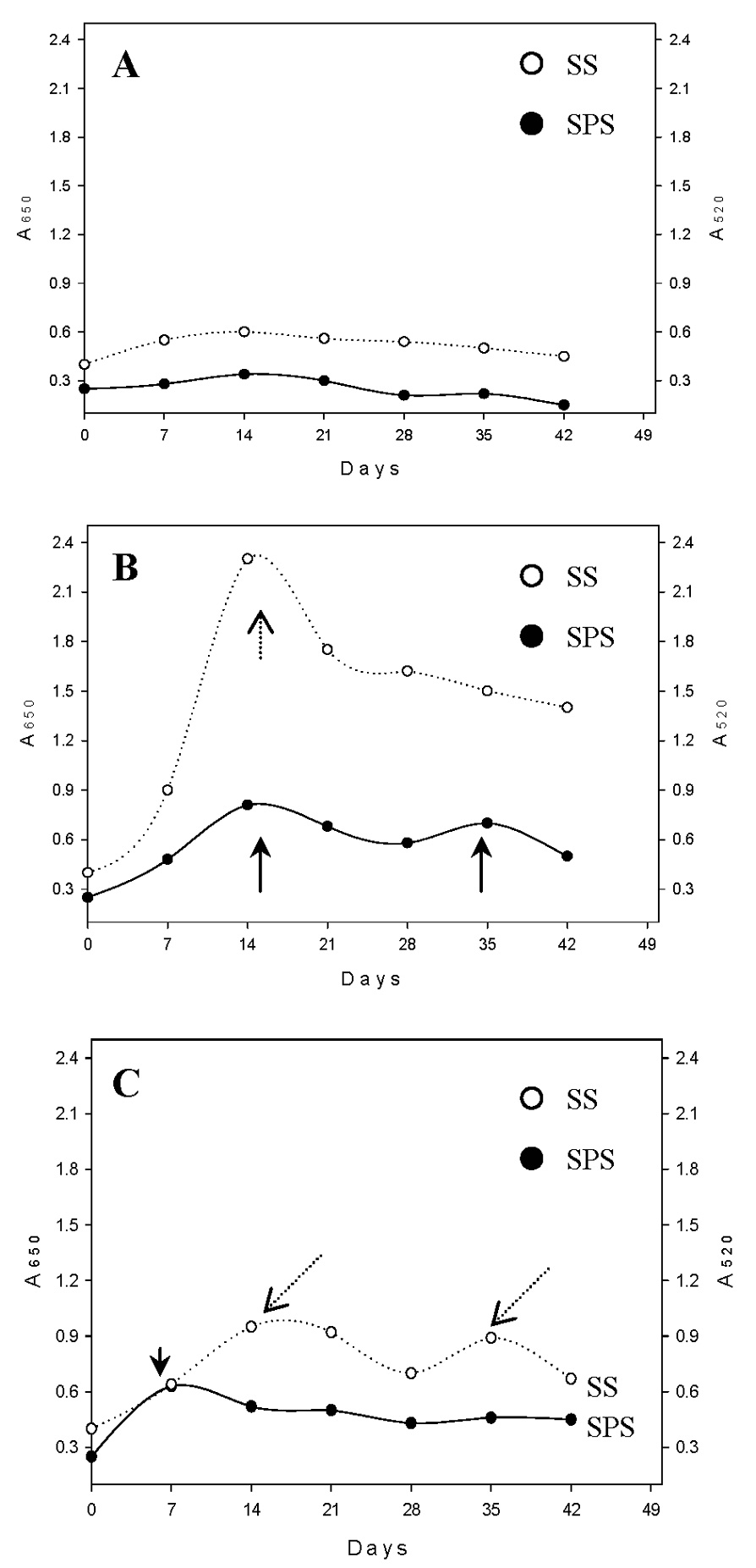
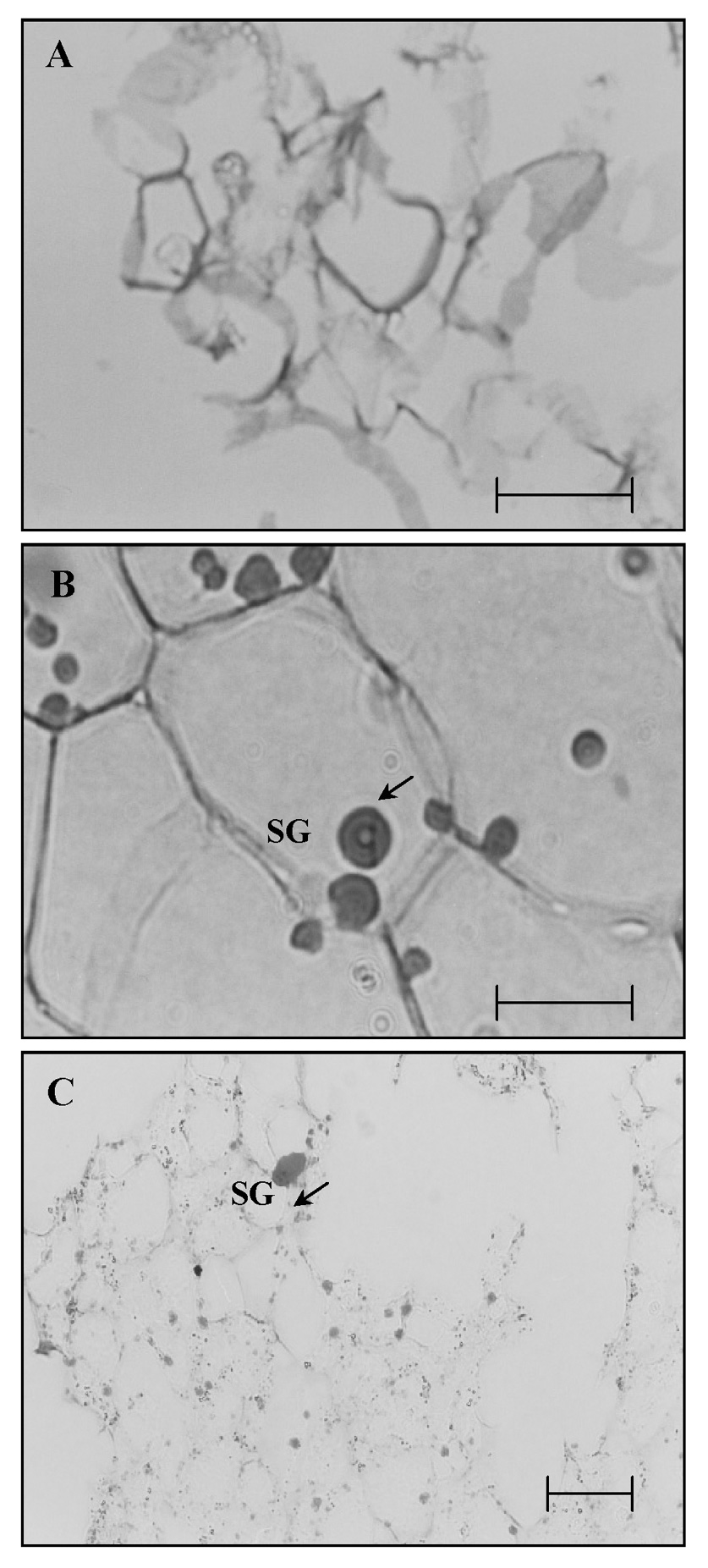
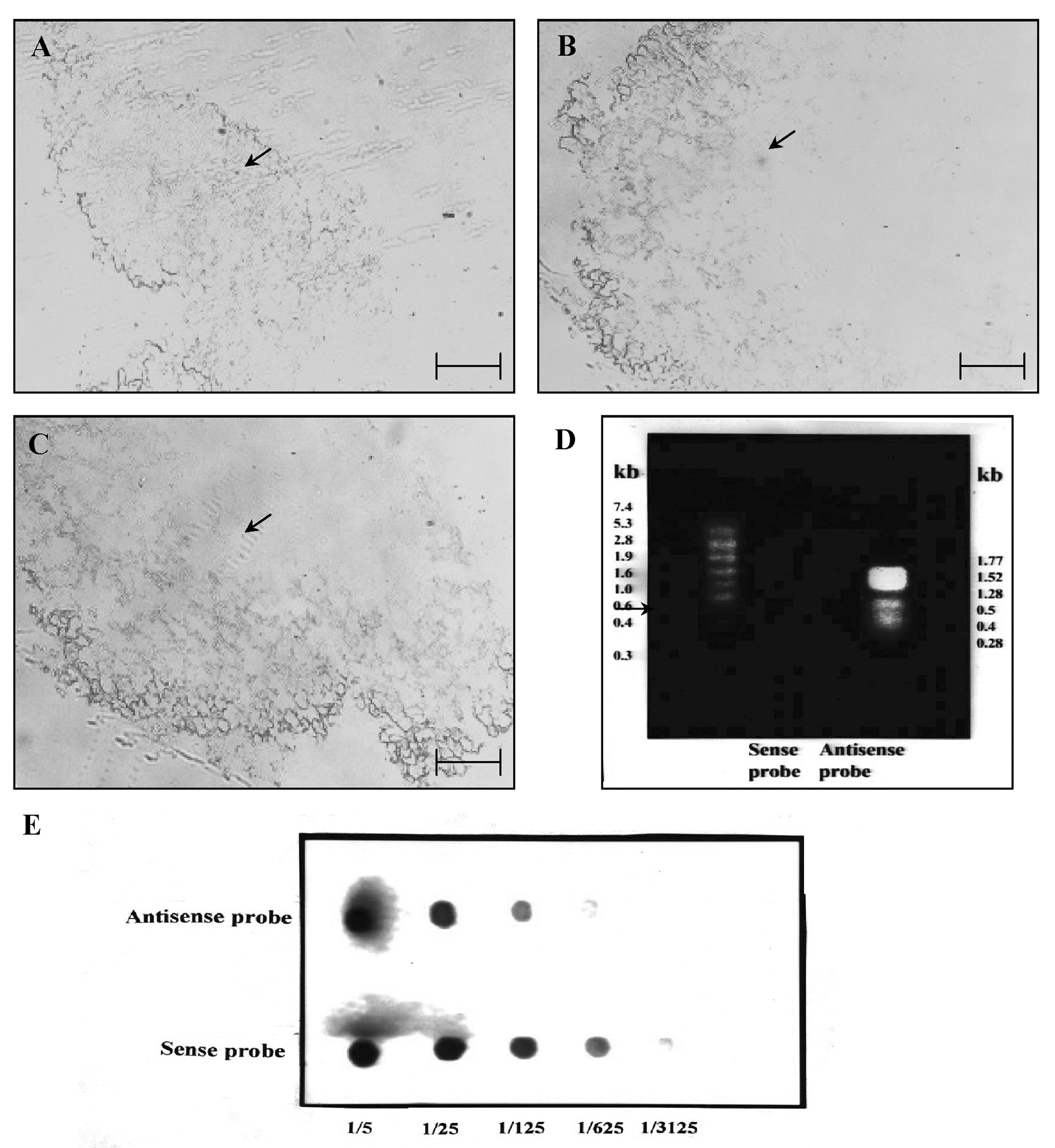
Abstract. Sucrose phosphate synthase (SPS) was purified from sweet potato callus grown under different conditions, including illumination, darkness, and osmotic stress. The properties of the enzymes purified from the different conditions were compared. Since sucrose synthase (SS) could also be purified using the purification process, the expression patterns and properties of the two enzymes were also compared. The SPS purified from the calli tissue medium under illumination had an optimum pH of 7.5. The enzyme had an optimum reaction temperature of 37°C, and maintained stable activity at pH 6 to 8. It was allosterically regulated using either Glc 6-P or Pi. However, the enzyme purified from the tissues grown under osmotic stress conditions was not allosterically regulated using Glc 6-P or Pi. The maximal activity of SPS and maximal protein content of the illumination grown calli appeared on the 14th day after the culture was transferred into a new medium. This was compared with the peak value on the 7th day for the samples under osmotic stress. The maximum activity for SS was observed on the 14th day. The dark grown cells had very low SPS, but normal SS activities. A histological study revealed an abundance of starch granules in the osmotic stressed cells. The in situ hybridization technique showed that the illumination and osmotic stress conditions induced the accumulation of SPS mRNA. The PAGE and enzyme activity assay data showed that, in addition to enhancing SPS gene expression, the activation of SS gene expression isozymes may be achieved under the illumination and osmotic stress conditions.
Keywords: Dark; Illumination; Osmotic stress; Sucrose phosphate synthase; Sucrose synthase; Sweet potato callus.
Introduction
Higher plants synthesize sucrose through two processes. The first and the major method employs sucrose phosphate synthase (EC 2.4.1.14; SPS), a soluble enzyme located in the cytoplasm that catalyzes the reaction:
UDPGlc + Fru6-phosphate UDP + Suc6'-phosphate
Suc 6'-phosphate is then dephosphorylated by Suc-P phosphatase (EC 2.3.1.14; SPP) to produce sucrose as the final product (Huber and Huber, 1996). The second is sucrose synthase (EC 2.4.1.13; SS) which catalyzes a reversible reaction:
UDP _ glucose + Fructose Sucrose + UDP
Sucrose is the main carbohydrate used by most plant species to translocate photo assimilates from the leaves to non-photosynthetic tissues. In addition, it is the most common carbon source added to plant tissue cultures. Sucrose is transported by a combination of symplasmic and apoplasmic pathways to the phloem, where it is loaded using a proton-driven symporter (Riesmeier et al., 1994). In the phloem, sucrose is translocated by a mass flow to sink tissues, where it is cleaved by SS to produce UDPG and fructose or it is hydrolyzed by invertase (EC 3.2.1.26) to
yield glucose and fructose (Giaquinta, 1983). They are then used for other biosynthetic pathways as energy and sources of structural components. SPS has also been proposed to be an important limiting step in the source-sink relationship, in grain filling in cereals (Prioul and Schwebel-Dugue, 1990), in sugar accumulation in fruits (Hubbard et al., 1990; Dali et al., 1992) and in cold stress acclimation (Guy et al., 1992).
SPS undergoes many regulatory mechanisms. First, SPS is subjected to allosteric regulation by Glc 6-P (activator) and Pi (inhibitor) (Douglas and Huber, 1983; Stitt et al., 1988). Secondly, it can be reversible and covalently modified in response to light (Stitt et al., 1988; Huber et al., 1989; Huber and Huber, 1991, 1992), accumulation of sucrose (Stitt et al., 1988) or osmotic stress (Toroser and Huber, 1997). Finally, it is involved with transcriptional regulation during sink-source transition (Harn et al., 1993; Klein et al., 1993).
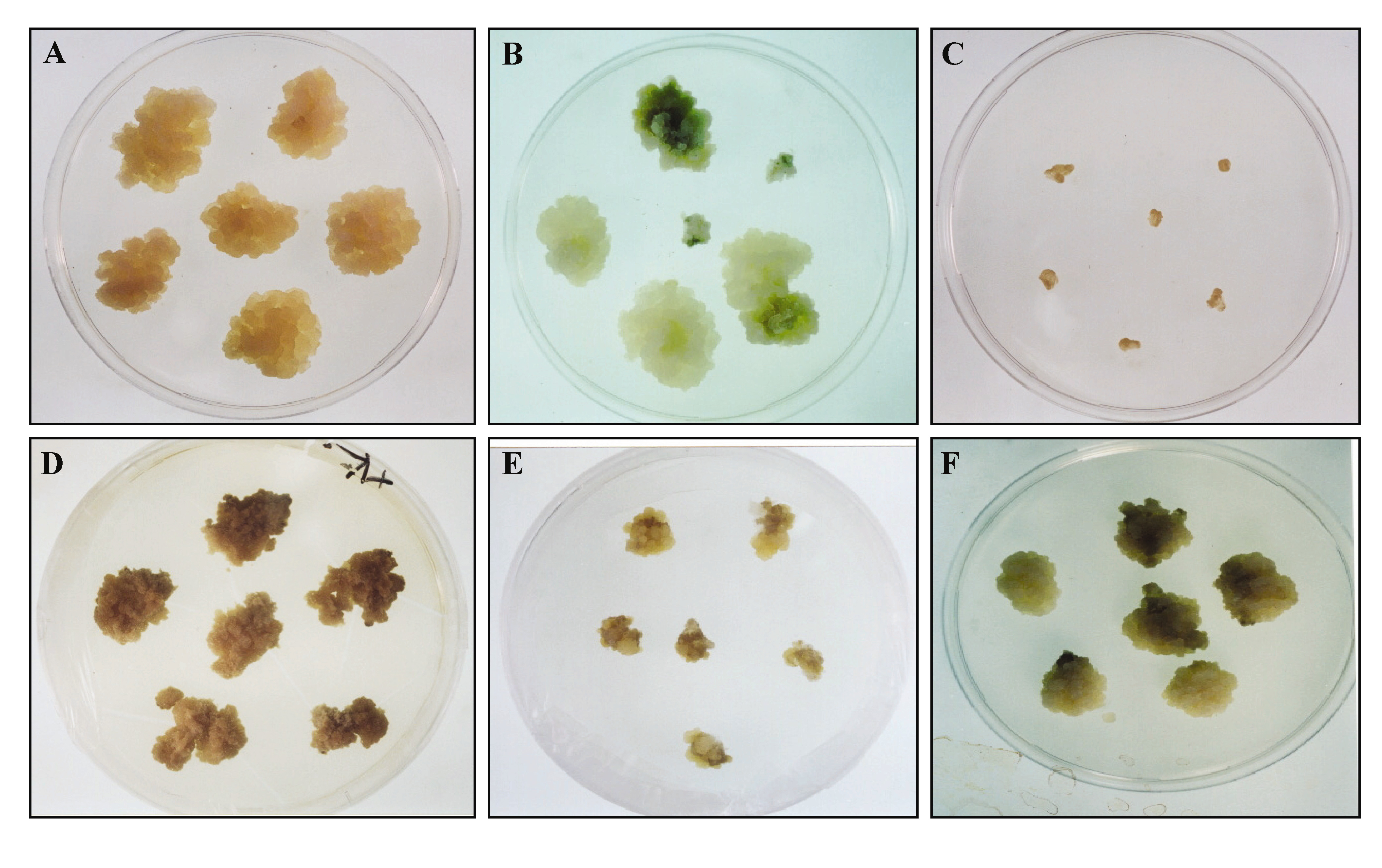
The aim of this report was to explore the SPS activity of non-photosynthetic tissues under illumination. The callus of sweet potato was induced from tubers for simplicity in manipulating the conditions. After treatment with illumination and high osmotic stress, the callus cells acquired a high starch content. Illumination and high osmotic stress were then used as two controlled factors for SPS activity. The condition of highest enzyme activity was used for the biochemical characterization of SPS.
*Corresponding author. E-mail: pingdu@ccms.ntu.edu.tw