Bot. Bull. Acad. Sin. (2003) 44: 297-303
Xie and Luo — Effect of leaf position and age on anatomical structure
Effect of leaf position and age on anatomical structure, photosynthesis, stomatal conductance and transpiration of Asian pear
Shenxi Xie1,2,* and Xianshi Luo1
1Department of Horticulture, Hunan Agriculture University, Changsha, Hunan 410128, P. R. China
2Department of Botany and Plant Science, University of California, Riverside, CA 92521-0124, USA
(Received November 20, 2002; Accepted May 27, 2003)
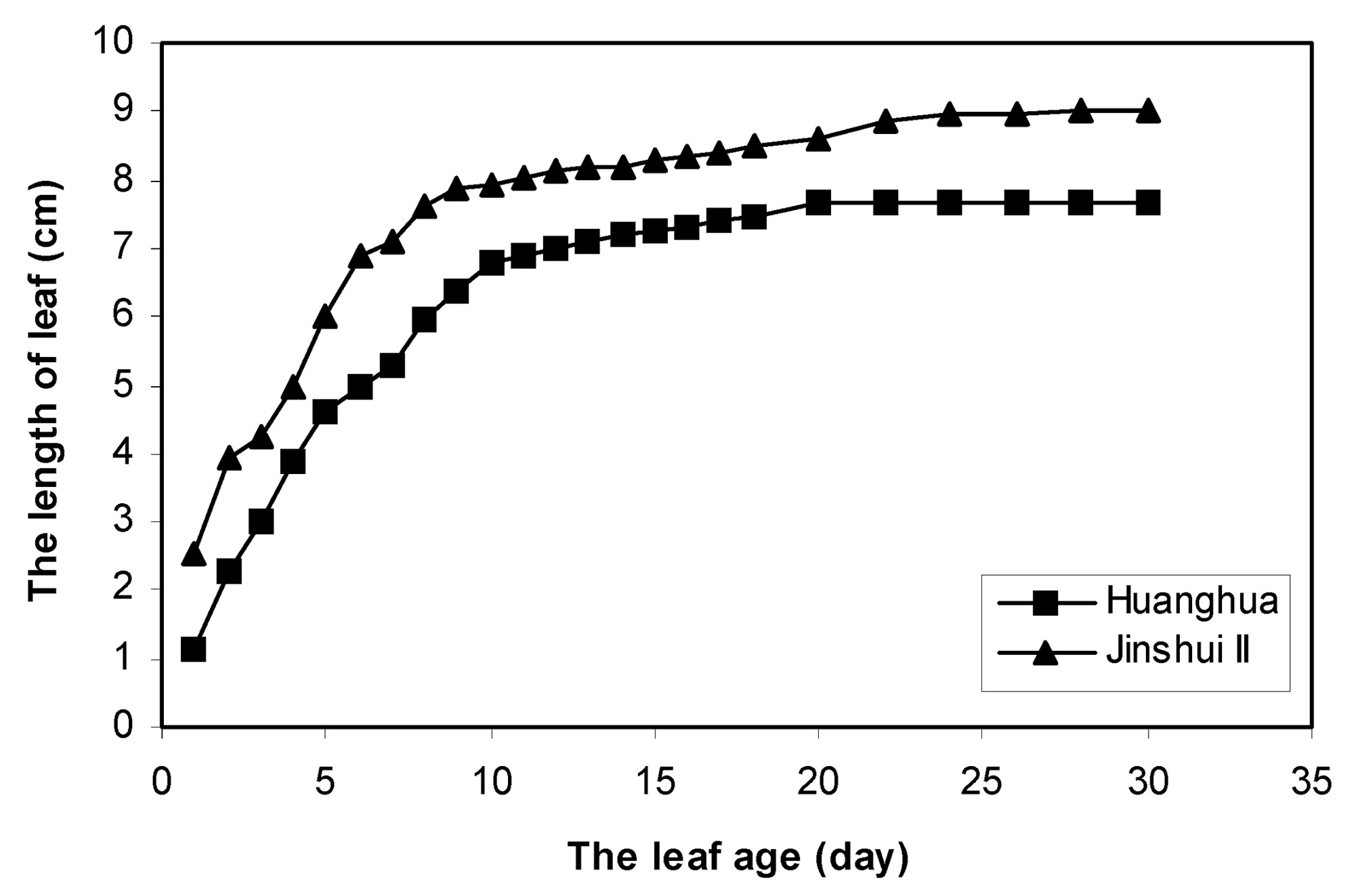
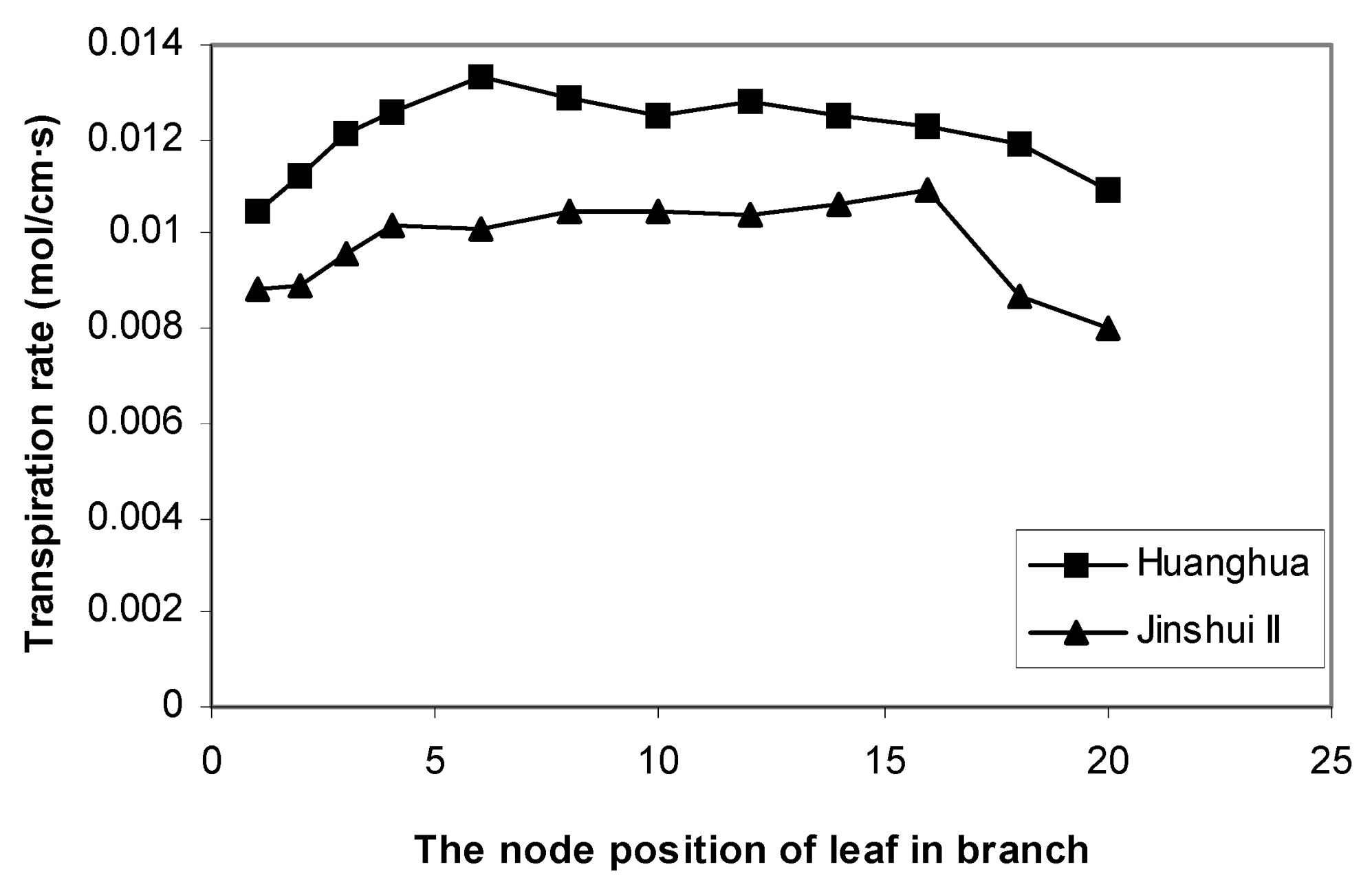
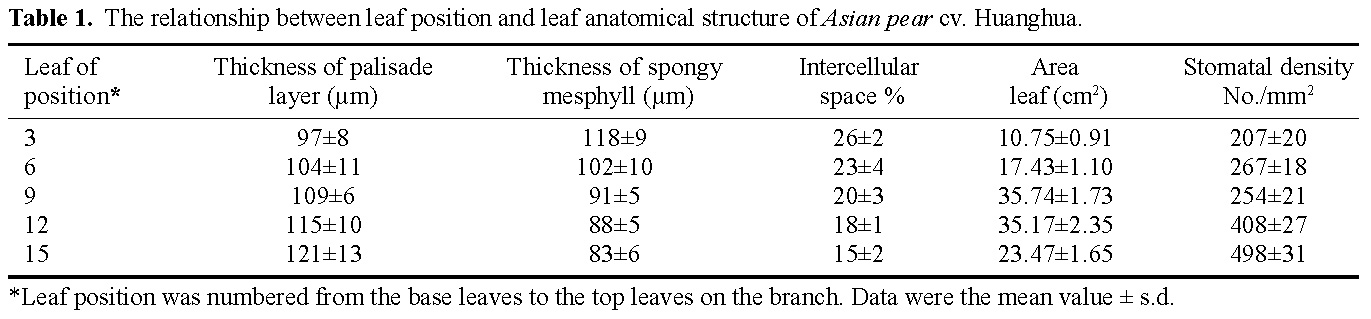
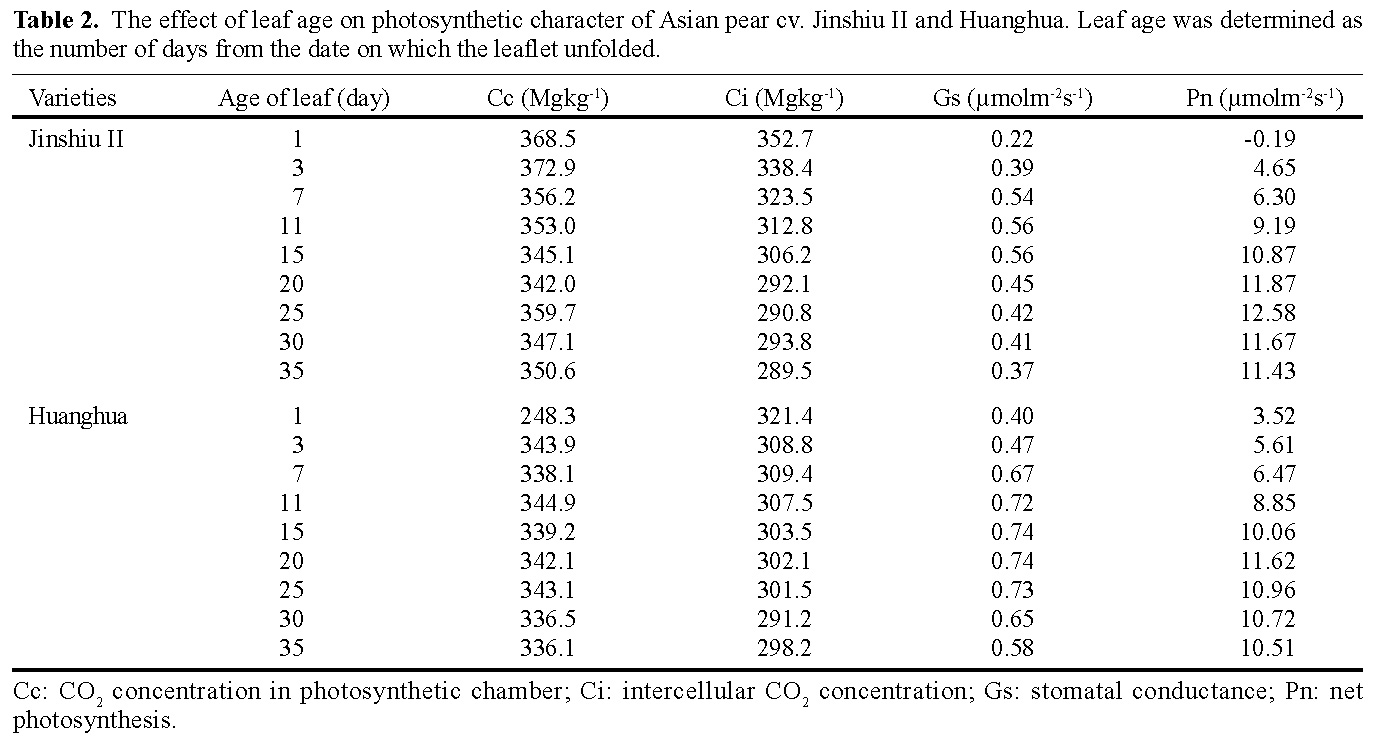
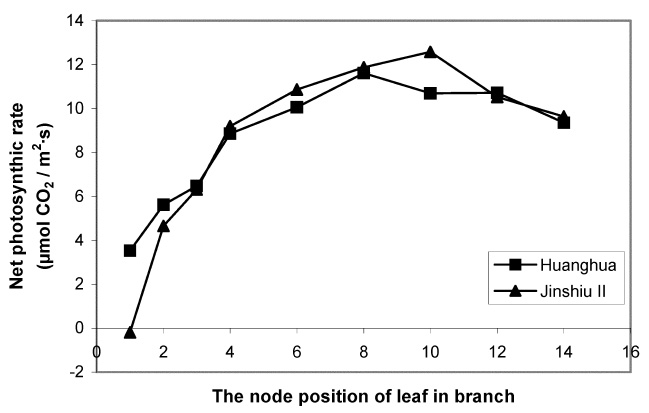
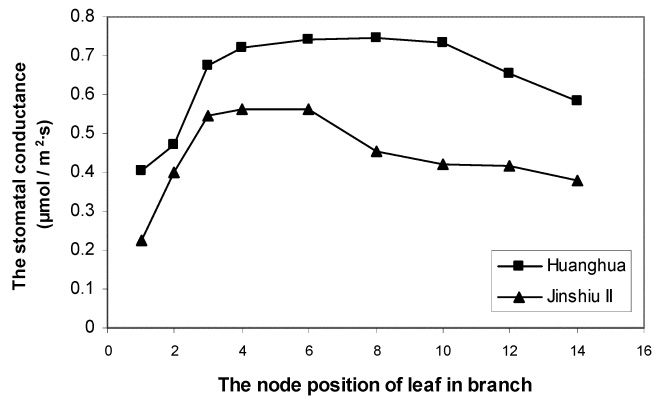
Abstract. The effects of leaf position and leaf age on gas exchange, transpiration, stomatal resistance, and anatomical structure of seven-year-old trees of Asian pear cv. Huanghua and Jingshiu II (Pyrus serotina Rehd. cv. culta rehd.) were examined under field conditions. Individual leaves were monitored from unfolding to maturation, and detailed measurements were made on leaves at various node positions and leaf ages. The anatomical structure varied according to the node position of the leaf, with palisade thickness increasing and the thickness of spongy mesophyll decreasing from the base to the apex of the branch. Intercellular space fraction of leaves decreased from the apex to the base of a shoot. "Huanghua" leaves took 20 days for full expansion while "Jingshiu II" required 25 days. Net photosynthesis (Pn) and stomatal conductance (Gs) of both genotypes increased with leaf age, particularly in the early stage. Pn reached a maximum value when the leaf was completely expanded. Leaves at different developmental stages from the apical position to the base performed differently. The transpiration rate and the vapor pressure deficit of older leaves on the base of the branch were higher than those of the younger leaves at the apex of the branch. Leaves at node 3 to 16 had higher saturation vapor pressure and transpiration rates. Both the apical and basal leaves had higher stomatal resistance and lower net photosynthesis than leaves in an intermediate position.
Keywords: Anatomical structure; Leaf position; Leaf age; Photosynthesis; Stomatal resistance; Transpiration.
Abbreviations: Pn, net photosynthesis; Gs, stomatal conductance; Ci, intercellular CO2 concentration; Rs, stomatal resistance; Rh, relative humidity; Cc, CO2 concentration in photosynthetic chamber; Tf, leaf temperature.
Introduction
Asian pear (Pyrus serotina Rehd. cv. culta rehd.) is one of the most prevalent tree crops in southern China. The subtropical climate of this region provides for cloudy, rainy conditions with low temperatures in the spring, when pears produce new shoots, expand leaves, flower and set fruit, increasing the demand for organic and inorganic nutrients. Plants meet these nutritional requirements three ways: (1) nutrient storage, (2) uptake of inorganic nutrients from the soil through the root system, and (3) synthesis of carbohydrate through leaves. Under unfavorable conditions, leaves have a stronger photosynthetic capacity to supply sufficient carbohydrates and organic nutrients to assure pears can finish various biochemical and physiological processes. Hancock and Flore (1989) reported the regression coefficient of net photosynthetic rates was significantly correlated with yield. Determining the leaf photosynthetic characteristics of pear is therefore important to maintaining yield and improving fruit quality.
Many studies have shown how environmental preconditioning such as temperature, light intensity, CO2
concentration, and soil water deficit affect stomatal response, gas exchange and photosynthesis (Mooney and Harrison, 1970; Berry and Bjorkman, 1980; Lieth and Pasian, 1990; Xie et al., 1996; Moriana et al., 2002). Based on changes during the day or between days, Heinicke and Childers (1937) concluded that light was the major factor affecting whole-canopy NCER of apple trees, and temperature was second most important, although Sirois and Cooper (1964) concluded CO2 was second. A recent study (Corelli-Grappadelli and Magnanini, 1993) reported short-term whole-canopy NCER measurements declined with gradually declining light levels for one apple tree. Photosynthesis and transpiration were greatly influenced by stomatal behavior. Stomatal opening is affected by CO2 concentration, vapor pressure gradient (VPG), light, turgor pressure caused by change in potassium and organic acids, and by abscisic acid (ABA) (Raschke, 1970; Schulze and Hall, 1982). Berry and Bjorkman (1980) found light intensity, CO2 concentration, temperature, and soil water deficit may have affected gas exchange of plants in a greenhouse. Bunce (1984) reported photosynthesis increased when humidity was increased in the environment. Transpiration at noon under a high temperature after 48 h of acclimation was 400% higher than under a moderate temperature. Stomatal resistance reached a minimum at noon, in accordance with the transpiration rate. Leaf wa
*Corresponding author. Tel: +001-9097874663; Fax: +001-9097874437; E-mail: shxi@citrus.ucr.edu