Bot. Bull. Acad. Sin. (2003) 44: 329-336
Souto and Premoli — Divergence among A. aurea populations
Genetic divergence among natural populations of Alstroemeria aurea D. Don: A dominant clonal herb of the understory in subalpine Nothofagus forests
Cintia P. Souto* and A.C. Premoli
Laboratorio Ecotono, Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, Quintral 1250, 8400 Bariloche, Argentina
(Received October 2, 2002; Accepted May 27, 2003)
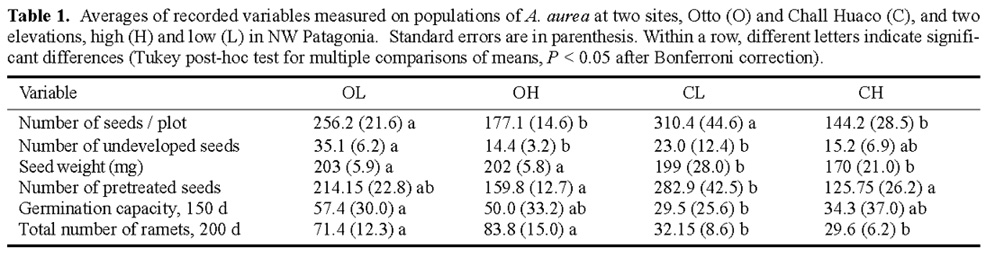
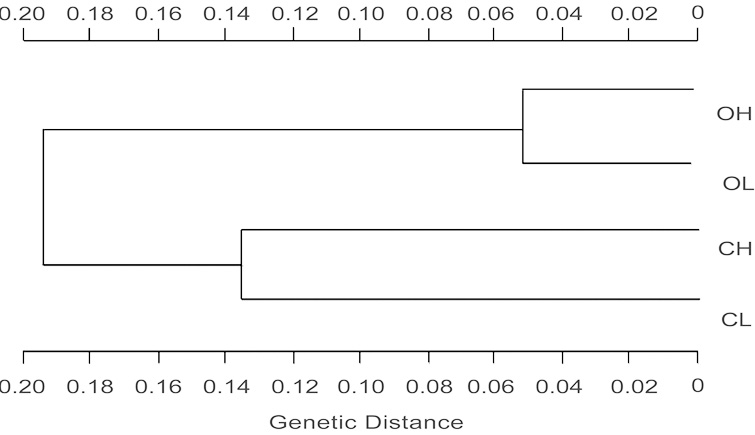
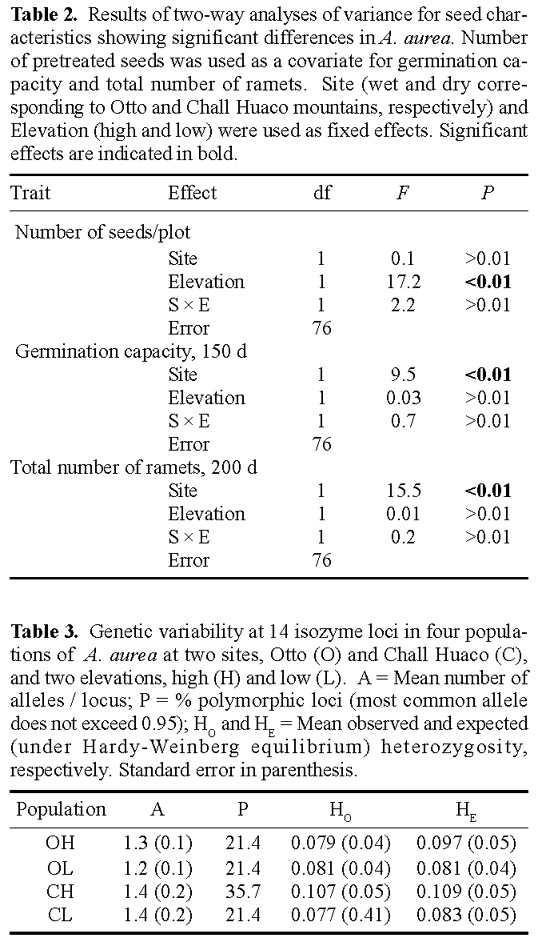
Abstract. Alstroemeria aurea is a perennial herb with clonal rhizomatous growth, insect pollination, and ballistic seed dispersal that inhabits a range of different environments in the southern Andes. We evaluated the hypothesis that differential selective pressures act together with restricted among-population gene flow result in genetically divergent populations of A. aurea. Study sites were located within Nahuel Huapi National Park (41°8'S, 71°19'W) at two mountain ranges and two different elevations. These were Chall Huaco Valley, a pristine site, with populations at 1,250 and 1,100 m, respectively, and Cerro Otto, a disturbed site, with populations at 1,250 and 950 m, respectively. Seeds at each of the four populations were harvested from 20, 1 × 1 m plots. These seeds were counted, weighed, and germinated after a cold and humid stratification for 4 months. Within- and between-mountain ranges differences in seed traits were evaluated by ANOVA. We genetically characterized 30 plants of each population by allozyme electrophoresis and estimated levels of genetic variation and divergence. Seed traits showed different responses to elevation and site conditions. Total number of seeds was greater at low-elevation populations even though they had a higher number of undeveloped seeds. Reduced seed yield at high-elevation populations may result from a short growing season at higher altitudes. Additionally, seed weight, germination rates, and early vegetative spread were significantly greater at Otto, which may suggest a selective strategy to colonize disturbed sites under favorable physical conditions. These between-site differences were supported by allozyme data. High genetic divergence, and thus low gene flow, was estimated among Otto and Chall Huaco whereas within each mountain range among-population divergence depended upon site characteristics. Higher gene flow rates were found in the disturbed site Otto. Our results indicate that restricted pollen and seed dispersal, together with selective forces acting in different habitats, may produce genetic differentiation in populations of A. aurea.
Keywords: Alstroemeriaceae; Allozyme electrophoresis; Gene flow; Genetic structure; Germination; Patagonia; Seeds.
Introduction
The ability of a plant species to occupy different environments could be the result of genetic adaptation, environmentally induced phenotypic plasticity, or a combination of these two processes (Jain, 1976). The relative importance of environment and genotype varies in the expression of a character. For example, differences in plant structure, leaf shape, and flowering time are often due to environmental factors while differences in pathogen resistance, flower architecture, and color are largely the result of genetic factors (Levin, 1984). Despite environmental influences, the genetic makeup of populations will directly affect their phenotypic characteristics such as the amount and kind of phenotypic variation, plant function including its interaction with the environment, and ecological tolerances of individuals together with their variation in time and space (Levin, 1984).
The amount and nature of genetic variation within and among populations is strongly affected by the mating system (Hamrick and Godt, 1990) and by the spatial relationship between plants and their parents (Hamrick and Nason, 1996). Populations where mating and seed dispersal occur over substantial spatial distances will have very different genetic properties as well as distinct ecological strategies from those in which these processes are restricted in space. Although random mating is a common assumption in many ecological and evolutionary models, it is unlikely to occur in plants, particularly when insect pollinators have restricted foraging behavior, such as bees tending to move from a plant to its near neighbors (Free, 1970; Levin and Kerster, 1974). In addition, for species with restricted seed dispersal, most progeny are established near their parents (Levin, 1984; Sobrevila, 1988; Redmond et al., 1989). Thus, the more restricted the dispersal of seeds and pollen, the stronger becomes the inverse relationship between distance and genetic kinship (Waser and Price, 1991; Souto et al., 2002). Spatial patterns of genetic variation for morphological, physiological, or phenological traits are the result not only of localized pollen and seed dispersal but also of different selection
*Corresponding author. Fax: +54-2944-422111; Phone: +54-2944-428505 Int. 508; E-mail: csouto@crub.uncoma.edu.ar