Bot. Bull. Acad. Sin. (2003) 44: 337-344
Chang et al. — Morphometric analysis of Kalopanax septemlobus
A morphometric analysis of the eastern Asian Kalopanax septemlobus (Thunb.) Koidz. (Araliaceae)
Chin-Sung Chang1, Hui Kim2,*, Ho-Sang Kang3, and Don Koo Lee3
1The Arboretum and Department of Forest Sciences and Products, Agriculture and Life Sciences, Seoul National University, san 56-1, Sillimdong, Gwanak-gu, Seoul, 151-742, Korea
2Korea National Herbarium (KH), Korea National Arboretum, 51-7 Jikdong-ri, Soheul-eup, Pocheon-si, Gyeonggi-do, Korea
3Department of Forest Sciences and Products, Agriculture and Life Sciences, Seoul National University, san 56-1, Sillimdong, Gwanak-gu, Seoul, 151-742, Korea
(Received September 17, 2002; Accepted June 24, 2003)
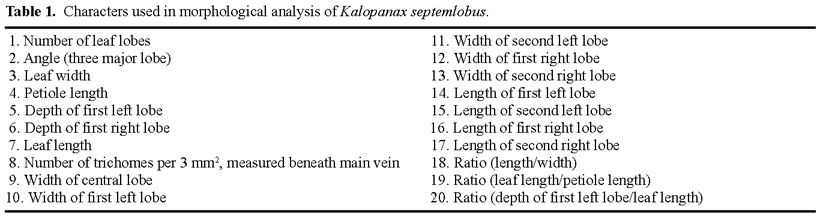
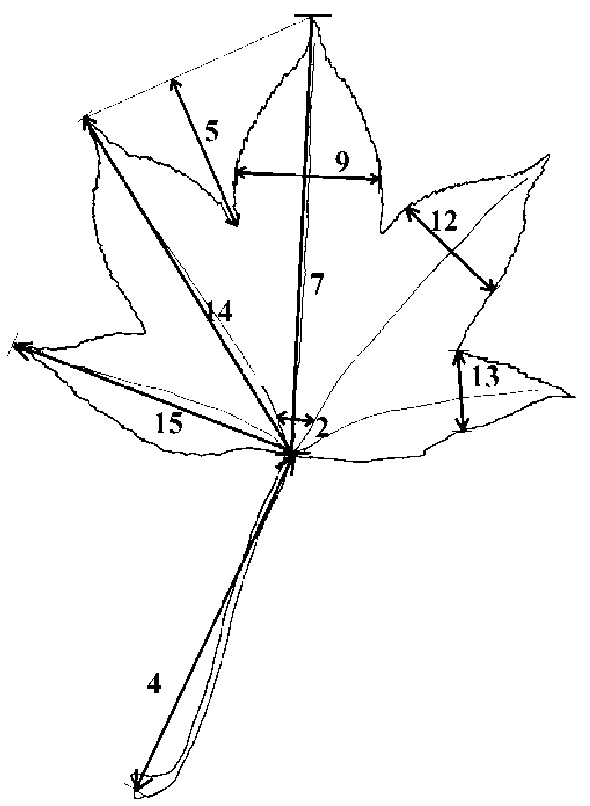
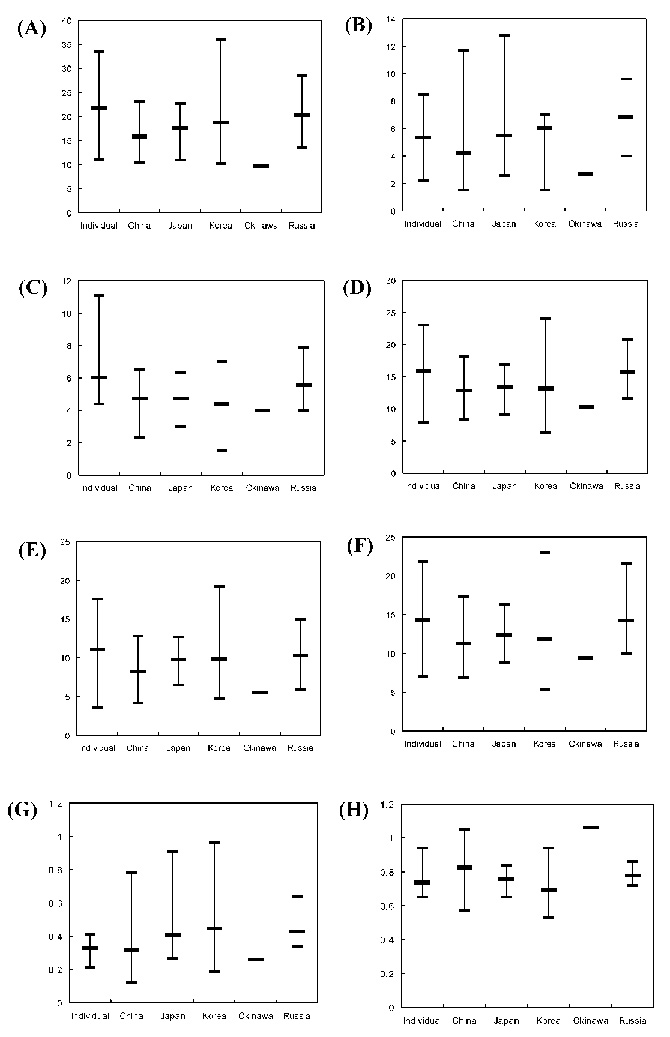
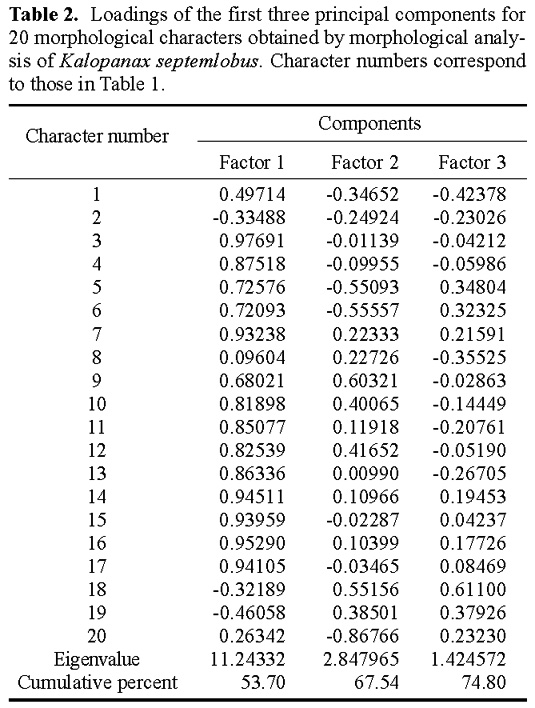
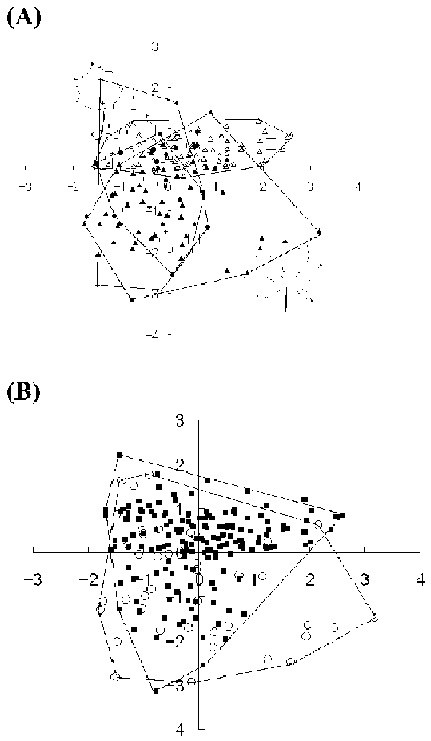
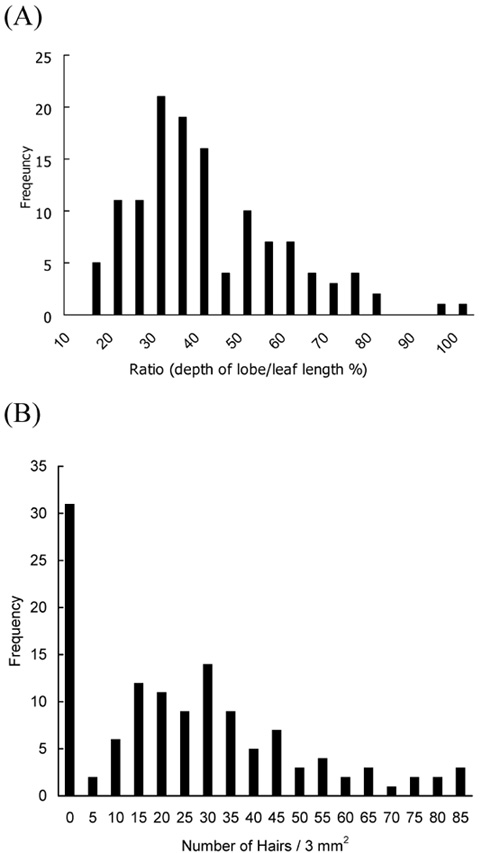
Abstract. Kalopanax septemlobus (Thunb.) Koidz., a woody species of Araliaceae, exhibits a broad range of morphological variation and occurs throughout much of eastern Asia. A morphometric analysis of herbarium material supplemented with a large sample of field-collected leaves was undertaken to determine if the morphological differentiation found within Kalopanax septemlobus warranted the taxonomic recognition of distinct taxa. One hundred twenty six individuals representing the total geographic range of the species were scored for 20 morphological characters, and the data matrix was subjected to principal components analysis. The results indicated that Kalopanax septemlobus should be recognized as one polymorphic species. Previously recognized infraspecific taxa were not supported to warrant the designation of any taxonomic rank. The observed pattern of variation may be environmentally induced and suggests that the species may exhibit environmental plasticity.
Keywords: Araliaceae; Forms; Kalopanax septemlobus; Kalopanax septemlobus subsp. lutchuensis; Morphometrics; Principal components analysis.
Introduction
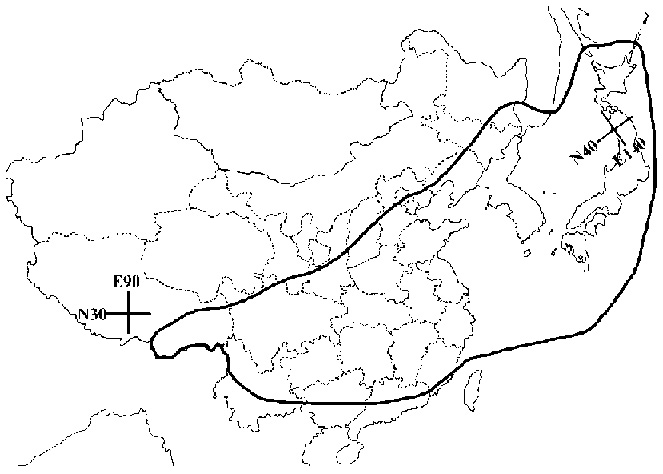
Castor Aralia, Kalopanax septemlobus (Thunb.) Koidz. (Araliaceae), is a well-defined species native to eastern Asia, Japan, Korea, China, and eastern coastal Russia (Willis, 1973) (Figure 1). Recent phylogenetic studies of the nuclear ribosom DNA internal transcried spacer (ITS) of Araliaceae have supported the monophyly of Kalopanax and Eleutherococcus and their placement in a broad assemblage comprising taxa largely with compound leaves (Wen et al., 2001).
Within the species, morphological variation is exhibited at many levels, from within a single population to among local and regional entities. Taxonomic treatments of this differentiation by previous workers (Nakai, 1927; Rehder, 1947; Lee, 1980; Hara, 1986) have recognized various numbers of infraspecific taxa at either the varietal or subspecific rank, usually based on minor differences in leaf morphology and trichoms which has made identification difficult. Zabel (in Ohashi, 1994) first noted that some of K. septemlobus was distinguished by tufted hairs on the basal axil of the main nerves, which he recognized as var. magnificus. Similarly, Van Houtte (1874) and Nakai (1927)
attempted to describe specimens with deeply incised leaves, which they designated as var. maximowiczii. However, Hara (1954) presented arguments favoring recognition of Nakai's variety as a form. Authors of regional flora treatments (Lee, 1980; Hoo and Tseng, 1978) have agreed with Nakai and Zabel's circumscriptions for the most part, maintaining the taxa at the varietal level. On the other hand, neither var. maximowiczii nor var. magnificus were
*Corresponding author. Tel: +82-31-540-1082; Fax: +82-31-540-1070; E-mail: huikim@chollian.net
Figure 1. Maximum extent of geographic distribution of Kalopanax septemlobus in eastern Asia.