Bot. Bull. Acad. Sin. (2003) 44: 345-351
Yukawa et al. — Reappraisal of Kitigorchis
Reappraisal of Kitigorchis (Orchidaceae)
Tomohisa Yukawa1,*, Shih-Wen Chung2, Yibo Luo3, Ching-I Peng4,*, Arata Momohara5, and Hiroaki Setoguchi6
1Tsukuba Botanical Garden, National Science Museum, Amakubo, Tsukuba, 305-0005, Japan
2Division of Forest Biology, Taiwan Forestry Research Institute, Taipei, Taiwan 100
3Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, 100093, China
4Institute of Botany, Academia Sinica, Taipei, Taiwan 115
5Faculty of Horticulture, Chiba University, Matsudo, 271-8510, Japan
6Faculty of Integrated Human Studies, Kyoto University, Kyoto, 606-8501, Japan
(Received April 21, 2003; Accepted September 10, 2003)
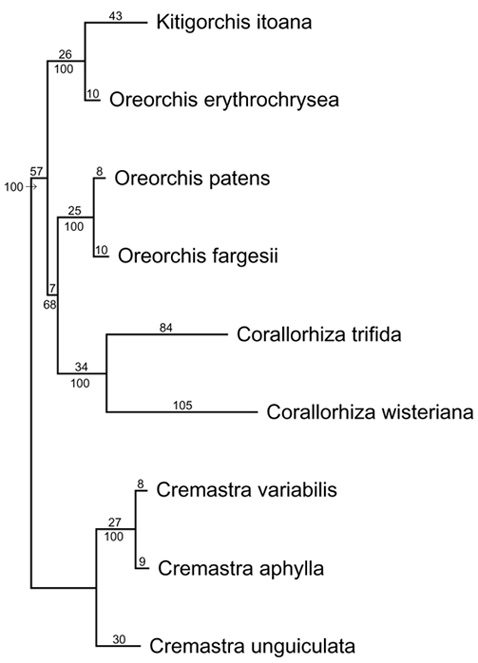
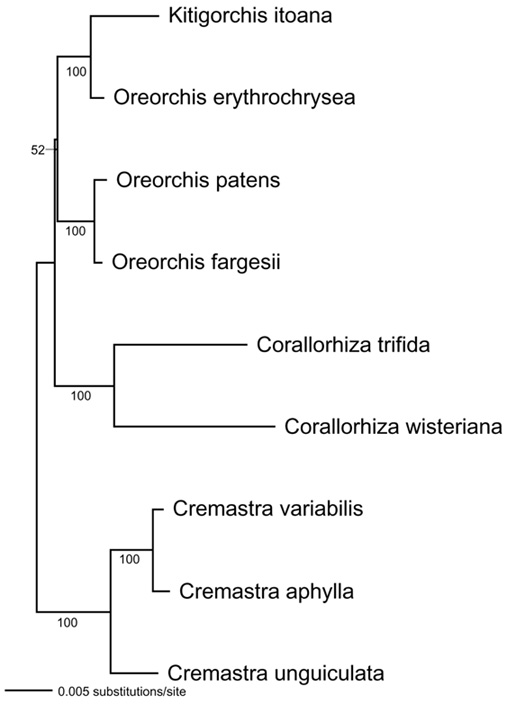
Abstract. Kitigorchis, a monotypic genus supposed to be endemic in the central part of Japan, was recently found in the Central Mountain Range of Taiwan. Further investigation of herbarium specimens and descriptions revealed that Kitigorchis itoana is conspecific with Oreorchis indica, previously recorded from the western Himalaya to southwestern China. This pattern of geographic distribution represents another example of disjunct distribution between Japan, Taiwan and the Himalayan region within the Eastern Asiatic Kingdom. Phylogenetic analyses using DNA sequence data from the internal transcribed spacer (ITS) region of the 18S-26S nuclear ribosomal DNA, matK, trnT-L intergenic spacer, trnL intron, trnL-F intergenic spacer, and rpL 16 intron showed that the monotypic Kitigorchis makes Oreorchis paraphyletic.
Keywords: Disjunct distribution; Japan; Kitigorchis itoana; Orchidaceae; Oreorchis indica; Phylogeny; Taiwan; Taxonomy.
Introduction
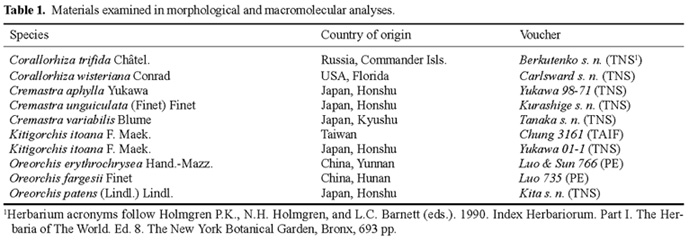
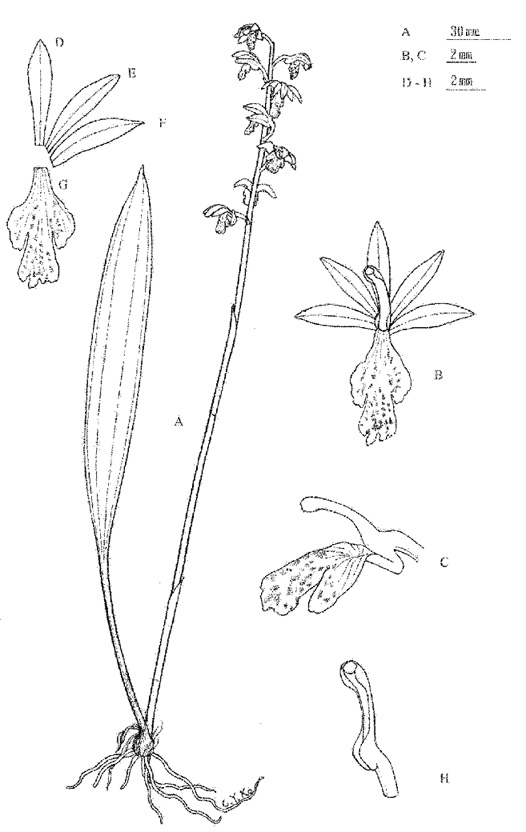
Maekawa (1971) established the orchid genus Kitigorchis on the basis of Kitigorchis itoana F. Maek. (Figure 1) together with Oreorchis foliosa (Lindl.) Lindl., Oreorchis erythrochrysa Hand.-Mazz., and Tainia shimadai Hayata. He separated Kitigorchis from Oreorchis by (1) the clustered, multi-branched rhizomes, (2) the distinct mentum, (3) the poorly developed calli on the lip, (4) the rigid leaf blade, and (5) the prominent nerves on the leaf. Subsequently, Hashimoto and Kanda (1981), Satomi (1982), Masamune (1984), Hashimoto (1987), Hashimoto et al. (1991), Imai (1997), Inoue and Ikegami (1997), and Environmental Agency of Japan (2000) followed Maekawa's treatment.
In their revisionary work of Oreorchis Lindl., Pearce and Cribb (1997) discussed the status of Kitigorchis and concluded that Kitigorchis is monotypic, comprising only K. itoana. They remarked that the clustered, multi-branched rhizomes of K. itoana are quite different from the subterranean organs of Oreorchis, but they did not recognize the other features used to separate these genera by Maekawa
(1971) as significant. They thus rejected Maekawa's transfer to Kitigorchis of the three taxa that do not form multi-branched rhizomes. They speculated that Kitigorchis is a genus intermediate between Corallorhiza R. Br. and Oreorchis. Since Maekawa's (1971) description of K. itoana was devoid of Latin diagnosis, it was not validly published. Throughout this paper, we provisionally use this invalid name because the development of the subject can be recognized correctly by use of this name and K. itoana has been used widely at least among literature in Japan.
In 2000 S.W. Chung and his associates found a terrestrial orchid in Taiwan that T. Yukawa confirmed to be Kitigorchis itoana (Figure 2). Previously, K. itoana was thought to be endemic in coniferous forests in a very narrow range in the central part of Japan on Mt. Yatsugatake, Mt. Komagatake, Mt. Karakitake, and Mt. Fujisan. This peculiar disjunct distribution led us to reexamine Kitigorchis.
Based on the results of a global phylogenetic analysis of the tribe Calypsoeae Dressler using matK, the maturase-encoding gene located in an intron of the plastid gene trnK (Yukawa, unpublished), Kitigorchis forms a clade with Oreorchis and Corallorhiza. Cremastra Lindl. is the sister group to the three genera and can be used as the outgroup for further analyses. As mentioned above, the generic status of Kitigorchis was based primarily on the
*Corresponding authors: Tomohisa Yukawa, Fax: +81-29-853-8998; E-mail: yukawa@kahaku.go.jp; Ching-I Peng, Fax: +886-2-2789-1623; E-mail: bopeng@sinica.edu.tw