Bot. Bull. Acad. Sin. (2004) 45: 55-60
Zheng et al. — Effects of C. laurentii on biocontrol of postharvest decay of arbutus berries
Effects of Cryptococcus laurentii (Kufferath) Skinner on biocontrol of postharvest decay of arbutus berries
Xiaodong Zheng*, Hongyin Zhang, and Yufang Xi
College of Biosystem Engineering and Food Science, Zhejiang University, Hangzhou, 310029, P.R. China
(Received July 16, 2003; Accepted September 16, 2003)
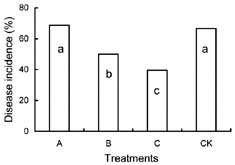
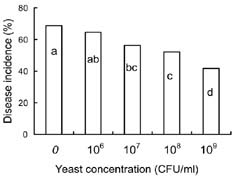
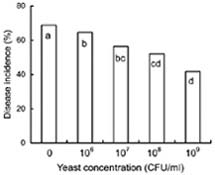
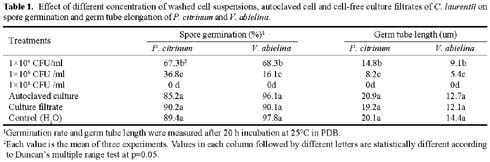
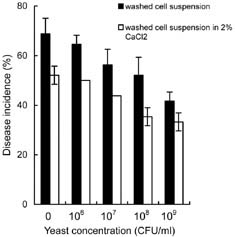
Abstract. Cryptococcus laurentii was tested as a biocontrol agent for reducing natural decay of arbutus berries caused by Penicillium citrinum and Verticicladiella abielina in semi-commercial postharvest trials. Three different preparations of C. laurentii were compared for antagonistic efficiency. Washed C. laurentii cells provided better protection against decay than yeast cultured in broth without washing while the culture supernatant free of yeast cells provided no protection. The protection provided by the washed yeast cells was dose-dependent. Cryptococcus laurentii was also effective in controlling decay at low temperature (4°C). The efficacy of C. laurentii was enhanced by the addition of 2% CaCl2. Agar disks of C. laurentii NYDA cultures placed on PDA plates seeded with pathogens did not inhibit the growth of P. citrinum or V. abielina. Spore germination of the pathogens in potato dextrose broth was strongly inhibited in the presence of active cell suspensions.
Keywords: Arbutus berry; Biocontrol; CaCl2; Cryptococcus laurentii; Postharvest decay.
Introduction
The shelf-life of the arbutus berry (Mycira rubra Sieb.et Zucc.) is very short because of its perishability and susceptibility to rot-causing pathogens. Postharvest pathogens cause major losses in arbutus berries in China. During storage and shipment of the arbutus berries, decay losses are mainly caused by Penicillium citrinum Thom and Verticicladiella abielina (Peck) Hughes. Synthetic chemical fungicides have been traditionally used to control these pathogens. Fungicide efficacy, however, is frequently decreased by the development of resistant strains of pathogens (Rosenberger and Meyer, 1981; Spotts and Cervantes, 1986). In addition, public concern and regulatory restrictions about the presence of fungicide residues on crops have emphasized the need to find alternative methods for disease control (Smilanick, 1994; Fan et al., 2000).
Biological control of fruit decay using a microbial antagonist has been considered a desirable alternative to synthetic fungicides. The control of major postharvest pathogens through application of biological agents was reported for stone fruits (Pusey and Wilson, 1984), pome (Janisiewicz, 1987, 1988; Janisiewicz et al., 1992), citrus (Chalutz and Wilson, 1990; Smilanick and Denis-Arrue, 1992), and other fruits (Lima et al., 1997).
Previous studies have shown that Cryptococcus laurentii (Kufferath) Skinner can be used as a bio-agent for postharvest control of gray and blue mold rot in apples (Roberts, 1990a), strawberries, kiwi fruits, and grapes (Lima et al., 1998), as well as of Mucor rot in pear (Roberts,
1990b). However, no literature is available on the postharvest biological control of mold rot in arbutus berries with a microbial antagonist. The objective of this study was to test whether C. laurentii can be used for postharvest biological control of mold rot in arbutus berries. We evaluated its effectiveness under semi-commercial conditions.
Materials and Methods
Fruits
The arbutus berries cultivars "Biji" were harvested at typical commercial maturity from Xianju of Zhejiang province. Fruits were used immediately after harvest.
Pathogen Inoculum
Penicillium citrinum and Verticicladiella abielina were isolated from infected arbutus berries and cultured on potato-dextrose agar medium (PDA: extract of boiled potatoes, 200 ml; dextrose, 20 g; agar, 20 g; and deionized water, 800 ml). Spore suspensions were prepared by removing the spores from the sporulating edges of a 2-3-week-old culture with a bacteriological loop and suspending them in sterile distilled water. Spore concentration was determined with a hemacytometer and adjusted as required.
Antagonist
The culture of C. laurentii was obtained from Institute of Microbiology, Chinese Academy of Science (Beijing, P. R. China). Yeast cultures were maintained at 4°C on nutrient yeast dextrose agar (NYDA: 8 g nutrient broth, 5 g yeast extract, 10 g glucose and 20 g agar, in 1 L of distilled water).
*Corresponding author. Tel: 86-0571-86971167; Fax: 86-0571-86045315; E-mail: xdzheng@zju.edu.cn