20
Botanical Studies, Vol. 51, 2010
promoter and introduced into the Agrobacterium tumefaciens strain AGL1. Arabidopsis was transformed by the floral dip transformation method (Clough and Bent, 1998). The transformants were selected on MS medium containing 40 fig/mL hygromycin.
Arabidopsis inflorescence grafting
Arabidopsis transformant stocks were grown for 4-5 weeks until bolting. The primary inflorescences of stocks were cut vertically 3-4 cm above the base. The scions were obtained from wild-type inflorescences with 2 to 3 lateral buds and cut into a V shape under microscopy. Both scion and stock were merged together with a piece of polyethylene tubing for physical support. The junction of the scion and stock was sealed with Parafilm and kept under high humidity for 7 days. The RNA samples were extracted at 14 days after grafting.
results
Phloem sap collection from broccoli (Brassica oleracea)
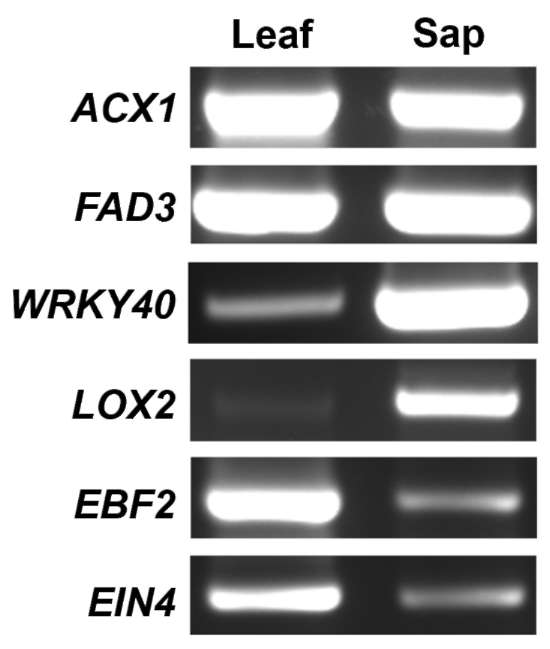
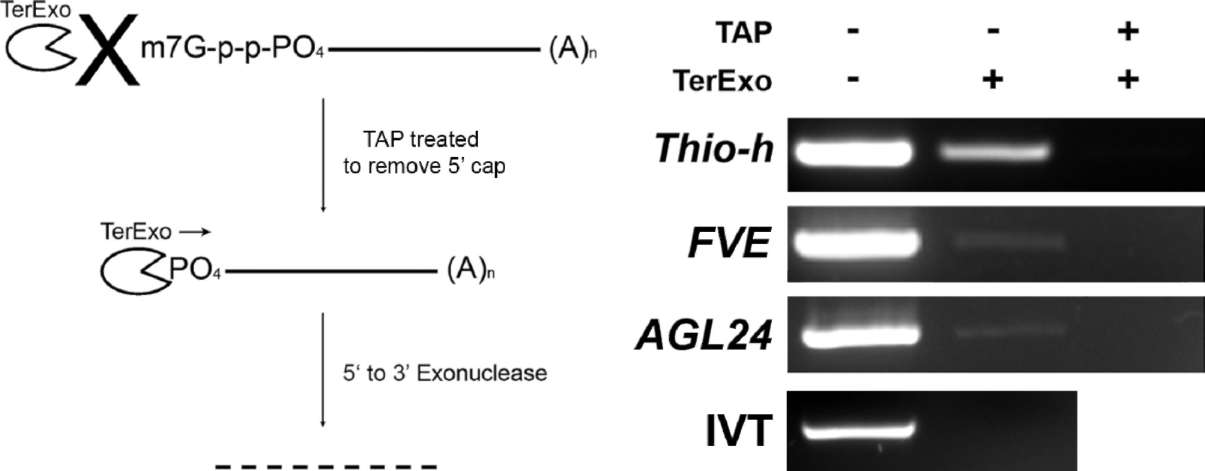
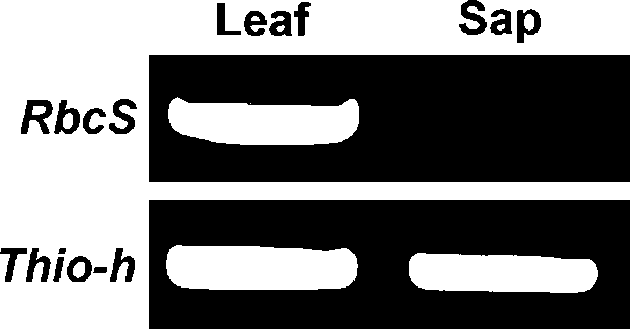
To investigate whether the RNAs of floral regulators are present in phloem sap, we chose broccoli (Brassica oleracea) as a system for phloem sap collection. Excised broccoli inflorescences showed high sucrose content (134.8±15.0 mg/mL) but low glucose content (5.7±0.1 mg/ mL) in exudates, which suggests that the exudates were largely derived from phloem. To test whether the exudates were contaminated by ruptured surrounding tissues during phloem sap collection, RT-PCR with primers of Rubisco small subunit (RbcS), a mesophyll-expressed gene, or Thioredoxin-h (Thio-h), a confirmed phloem-sap RNA (Giavalisco et al., 2006; Sasaki et al., 1998), were used. RT-PCR with Thio-h primers obtained signals from both phloem-sap and leaves (Figure 1). In contrast, when RbcS primers were used for RT-PCR with 30 cycles, the PCR products were detected only in RNA extracted from leaves but not phloem sap (Figure 1). However, when PCR was conducted with 35 cycles, a weak signal was detected from phloem sap sample (data not shown, Figure 2).
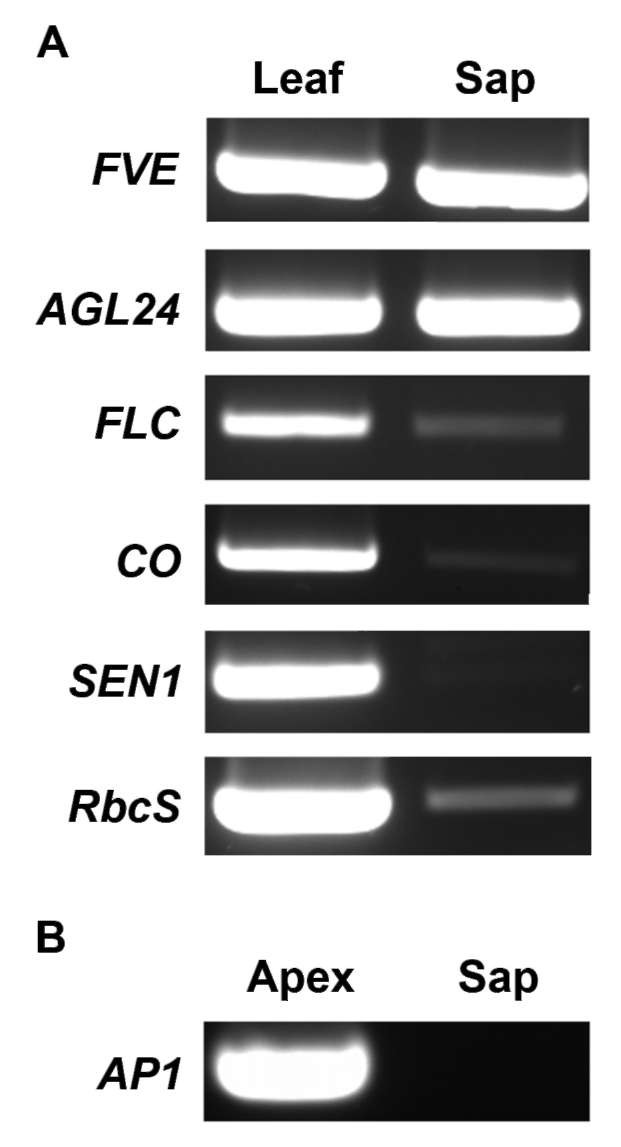
Figure 2. RT-PCR analysis of phloem-sap RNA. Total RNA was extracted from broccoli phloem sap and mature leaf (A) or flower apex (B).
Thus, although the phloem sap was contaminated by the surrounding tissues, the contamination was relatively low abundant.
Detection of floral regulators RNA in phloem sap
To analyze whether the RNA of floral regulators are present in the phloem sap, the broccoli phloem-sap RNA was hybridized with Arabidopsis ATH1 GeneChip. As shown in Table 2, the hybridization intensity (represented as arbitrary unit, the definition of the arbitrary unit was described in the materials and methods) of FVE and AGL24 in the phloem sap was significantly higher than that of other floral regulators, which suggests the presence
of FVE and AGL24 RNAs in the phloem sap.
To further verify whether the RNAs of FVE and AGL24 were present in the phloem sap, RT-PCR was conducted. When PCR was performed with 35 cycles, the signals
Figure 1. RT-PCR analysis of RNA extracted from broccoli leaf and phloem sap with use of gene-specific primers for Rubisco small subunit (RbcS), or Thioredoxin-h (Thio-h).