MOU and ZHANG 一 Rubovietnamia nonggangensis, a new species from China
121
RESULTS AND DISCUSSION
Morphology
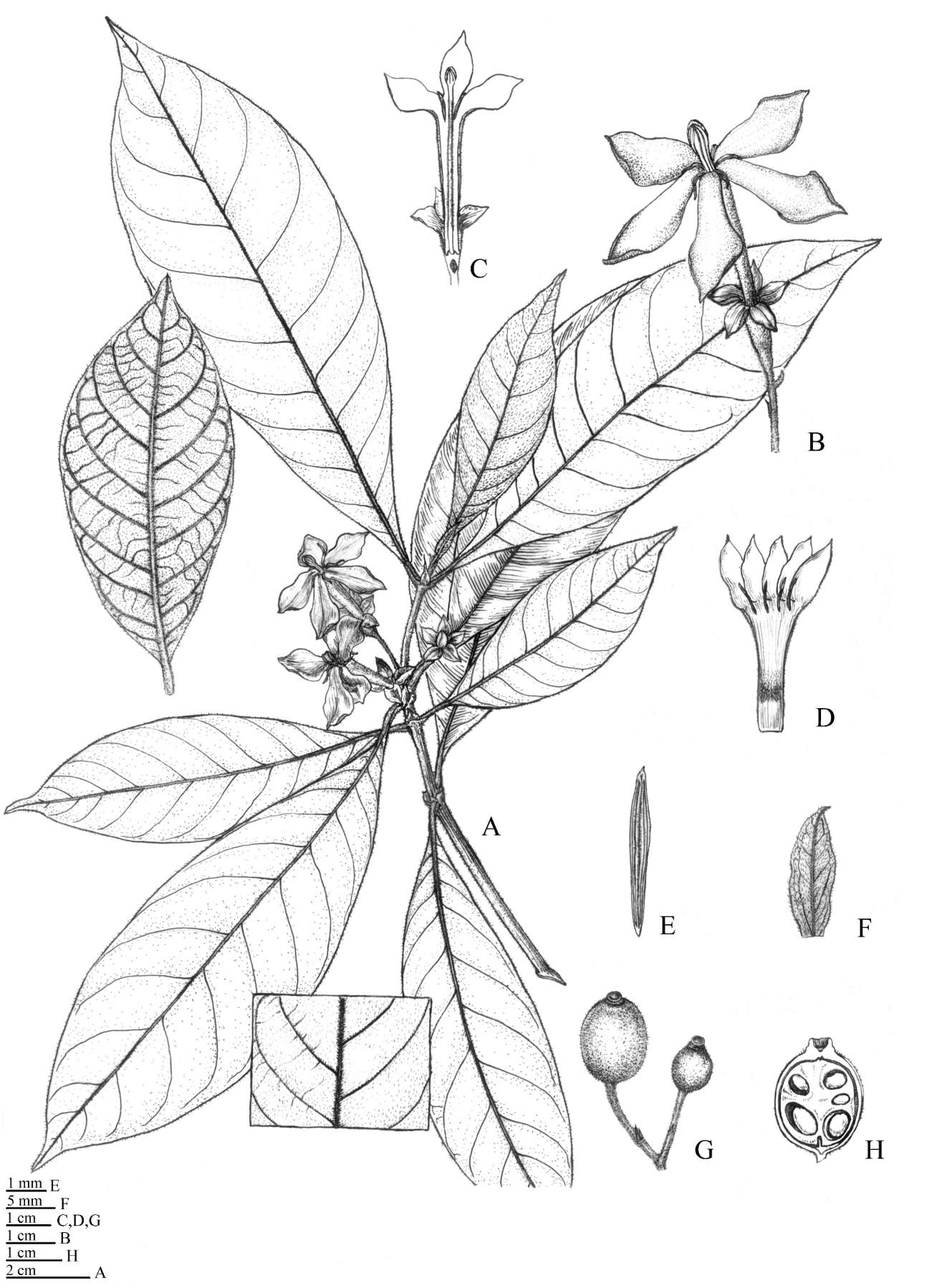
The collections from Guangxi Province bears a superficial resemblance to Rubovietnamia aristata Tirveng. in having cymose inflorescences, a well-developed corolla tube and lobes and globose fruits (Figure 1A-G; Table 2). However, they can be distinguished easily from R. aristata in having much broader foliaceous calyx lobes, and the leaves, young branches and flowers (including pedicles, ovary, styles and calyx) densely covered with hairs. These specimens are thus proposed as a new species here.
Palynology
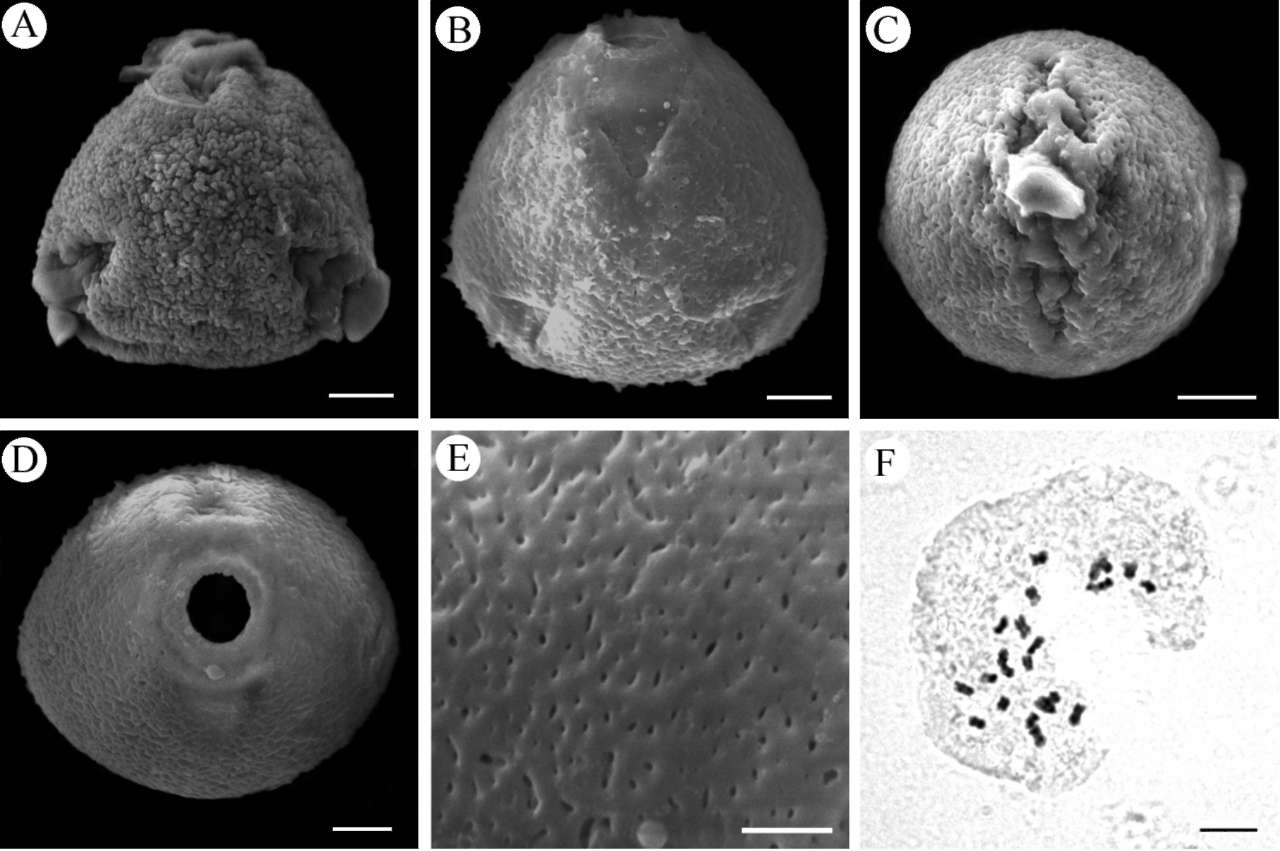
The pollen grains of the new species are monads, oblate, 23.72 士 0.82 (22.1-25.3) x 24.09 士 1.93 (21.3-26.0) [im in size, 3-colporate and the perforate or foveolate sexine ornamentation with perforations of variable size. Apertures are c. 5.7 x 4.6 [m in size, with prominent pro-
trudings (Figure 2A-E). The pollen grains of the new species are thus distinguished from Rubovietnamia aristata, which has heterogeneously reticulate sexine ornamentation (Tirvengadum, 1998). The exine ornamentation of pollen is usually reticulate, but also foveolate, rugulate, perforate and psilate in the subtribe Gardeniinae (Persson, 1993).
Cytology
The mitotic chromosome number of the new species is 2n = 22 (Figure 3F), which is congruent with that of most taxa reported in the tribe Gardenieae, including the genera Catunaregam, Massularia, Rosenbergiodendron, Rothmannia (Gadella, 1982; Kiehn, 1985; Kiehn and Lor-ence, 1996), Sukunia (Kiehn, 1996.) and Gardenia (Bhat-tacharyya, 1958), while 2n = 20 or 22 have been reported in Genipa (Guerra, 1993; Pierozzi and Mendagolli, 1997).
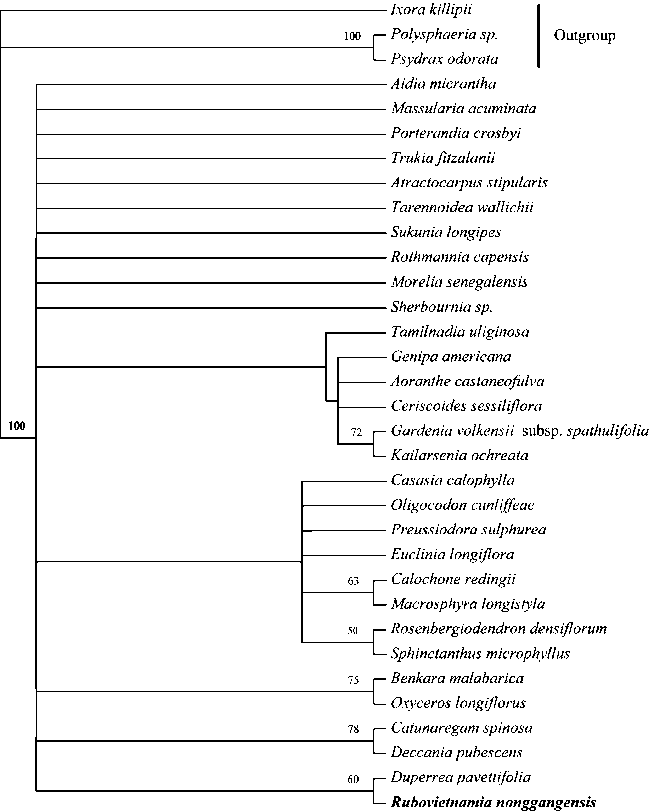
Molecular phylogeny
Each of the sequence addition replicates of the heuristic
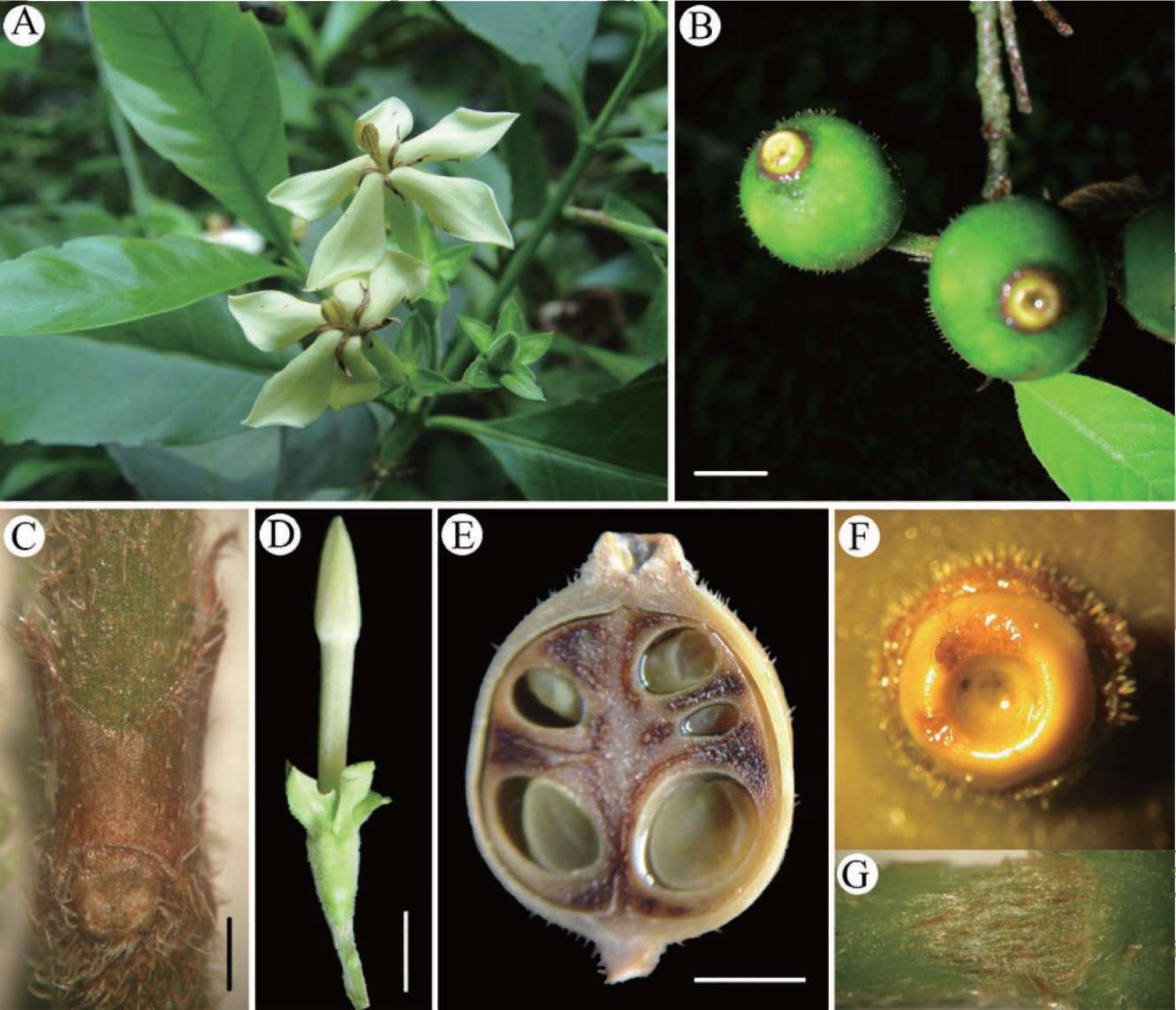
Figure 1. Rubovietnamia nonggangensis. A, Flowers; B, E, Fruits; C, Stipule; D, Bud; E, longitudinal section of a fruit; F, Floral disk on the fruit apex; G, Colleter on stem.