|
|
Morphological Study. The measurement of roots, petiole, rachis, scales and indusia was conducted with a micrometer under microscope.
Spore Morphology. The spore samples were taped onto a specimen stub with double-sided tape and sputter-coated with gold-palladium. Observations were conducted using a JSE-5900LV Scanning Electron Microscope (SEM) (Electron Co., Tokyo, Japan) at 20 kV at Sichuan University, Chengdu, China. Measurements were carried out using digital images of 20 spores with the measure tool in Adobe Photoshop (ver. 7.0.1; Adobe Systems Inc., San Jose, California). Descriptive terminology of the spores follows Punt et al. (2007) available online (http://www.bio.uu.nl/~palaeo/glossary).
Molecular Methods. Total genomic DNA was isolated from silica-dried leaves using Plant Genomic DNA
Kits (TIANGEN BioTech., Beijing, China). The plastid
trnL-F intergenic spacer was amplified with the universal primers e and f of Taberlet et al. (1 991 ). The PCR protocols followed Zhang et al. (2001) and Zhang and Renner (2003). Amplified fragments were purified with TIANquick Midi Purification Kits (TIANGEN). Purified PCR products were sequenced by InvitrogenTM (Shanghai, China). Most DNA sequences were downloaded from GenBank.
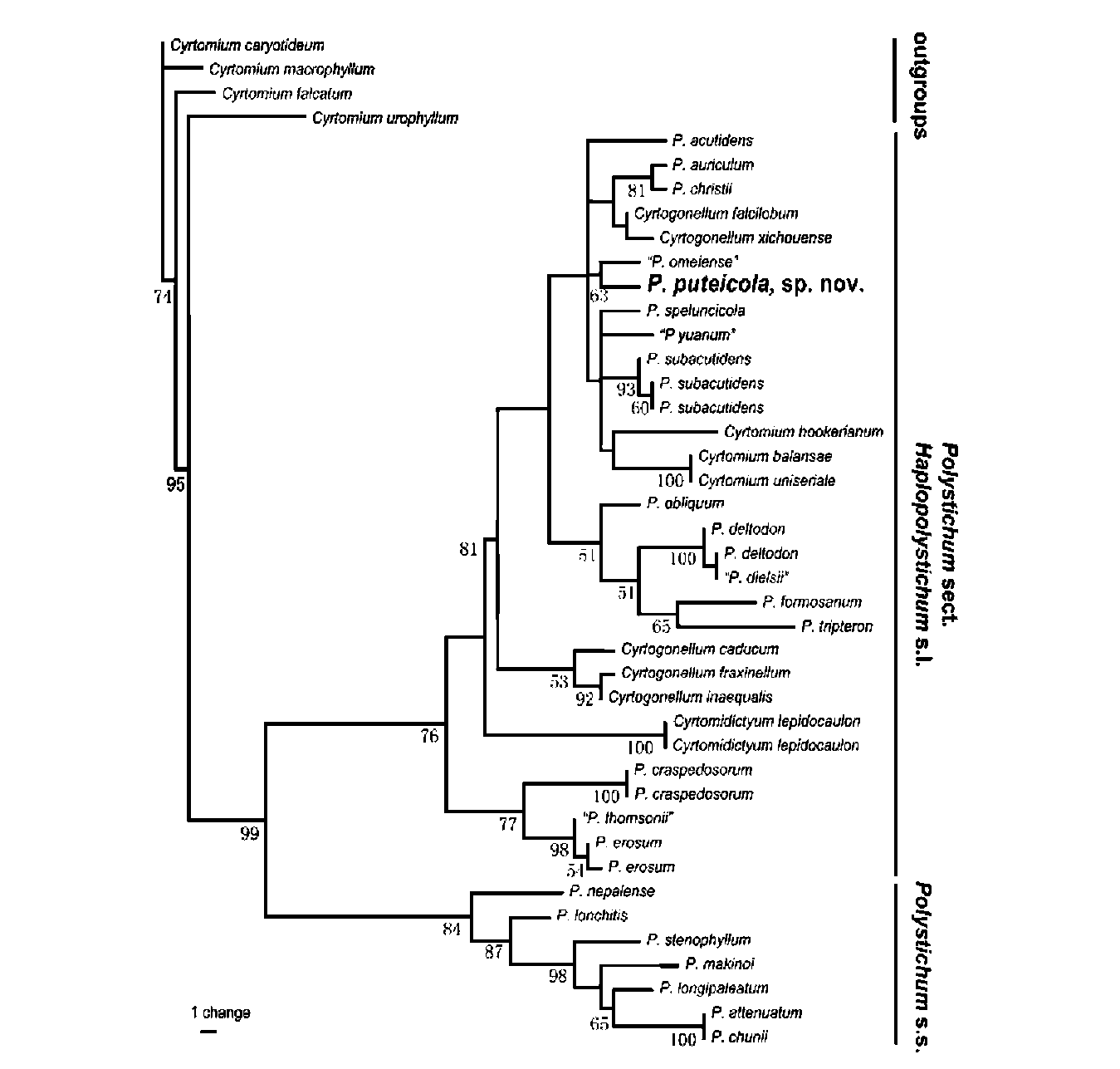
Twenty-five species of sect. Haplopolystichum s.l. (Zhang and He, 2009a) and seven representatives of the monophyletic Polystichum s.s., as resolved in Driscoll
and Barrington (2007), Lu et al. (2007), and Li et al.
(2007, 2008), constituted the ingroup. Four species of Cyrtomium s.s., i.e. C. caryotideum (Wall.) Presl, C. falcatum (L. f.) Presl, C. macrophyllum (Makino) Tagawa, and C. urophyllum Ching, were used as outgroups based on Lu et al. (2005) and Li et al. (2008). A few species were represented by more than one accession. In total 42 accessions were included in the analysis. All sequences used in this study together with their GenBank accession numbers and voucher information or source publications are listed in Appendix I. Preliminary alignment of nucleo-tides was obtained using the default alignment parameters in Clustal X (Thompson et al., 1997) followed by manual adjustments. Gap characters were scored using modified complex indel coding (Simmons and Ochoterena, 2000; Muller, 2006).
Phylogenetic analysis followed the procedure in Zhang and Simmons (2006) and Zhang and He (2010). Equally weighted parsimony tree searches were conducted using 1,000 tree-bisection-reconnection (TBR) searches in
PAUP* 4.0b10 (Swofford, 2001) with a maximum of 1,000
trees held per TBR search. Parsimony jackknife analyses (Farris et al., 1996) were conducted using PAUP* with the removal probability set to approximately e"1 (36.946%), and "jac" resampling emulated. One thousand replicates were performed with ten TBR searches per replicate and a maximum of 100 trees held per TBR search.
|
RESULTS AND TAXONOMIC TREATMENT
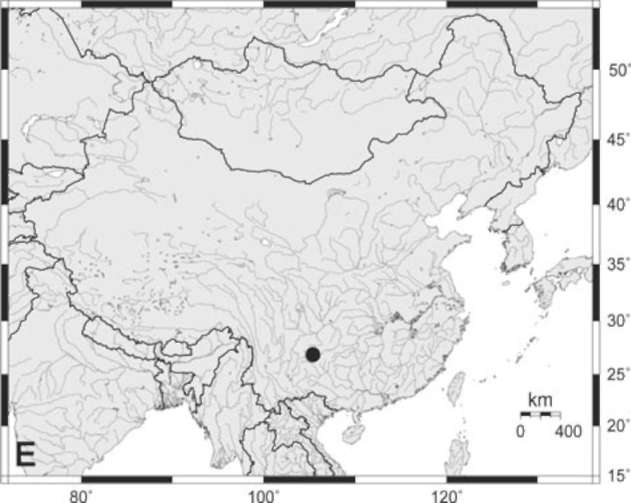
Polystichum puteicola L. B. Zhang, H. He & Q. Luo, sp. nov.-TYPE: CHINA. Guizhou Province: Bijie City, Fangzhu Town, Baini Village, Tuntianjing, 27°14.42' N, 104°59.06' E, on limestone wall, 0.5-1.5 m above the bottom of a sinkhole, c. 200 m under the ground, alt.
1,720 m, 17 Oct. 2008. L. B. Zhang, H. He, Q. Luo & C. B. Jiang 706 (HOLOTYPE: MO; ISOTYPES: CDBI,
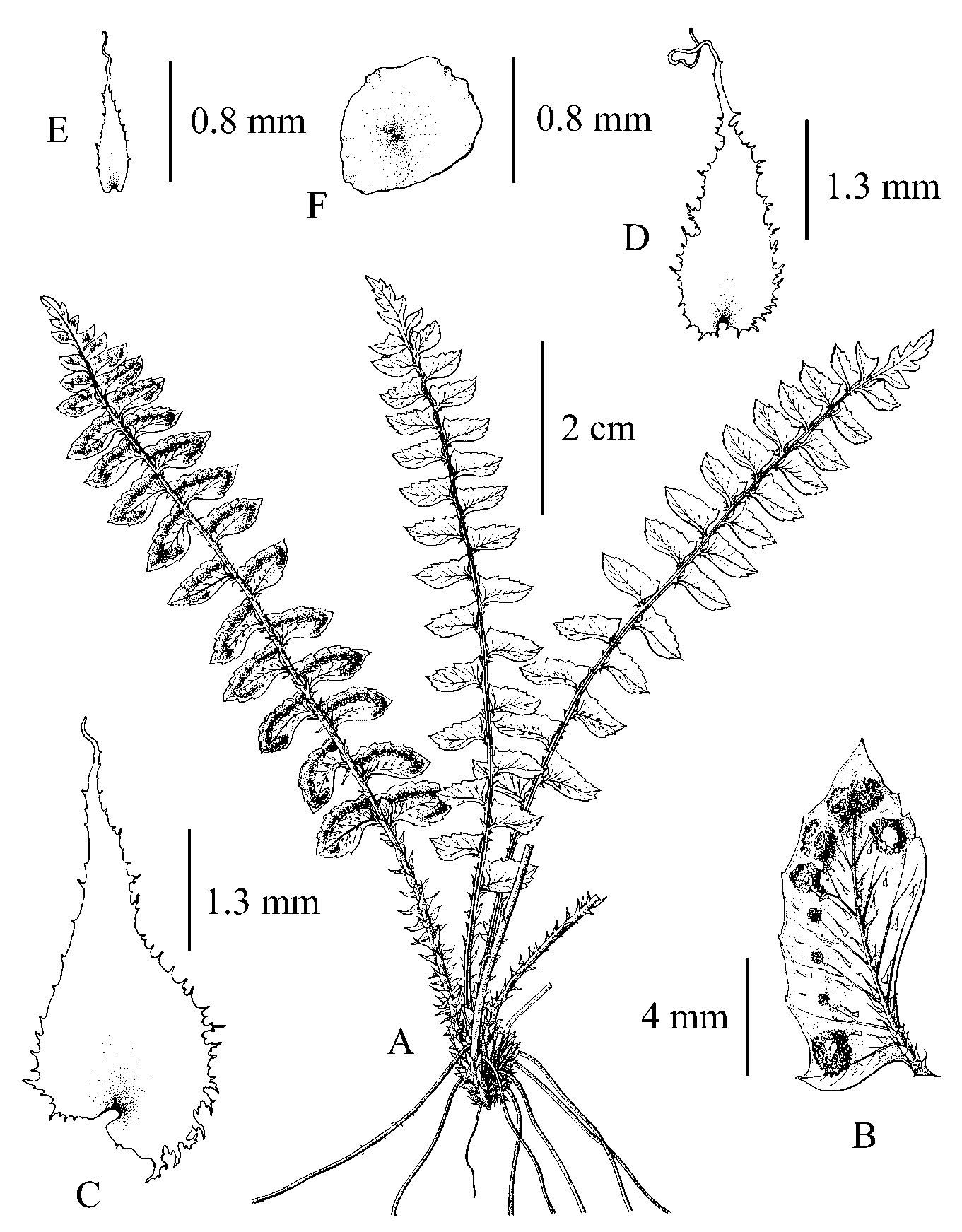
CTC, HAST, MO).吞天井耳蕨 Figures 1, 2
Species valde affinis P. obliquo (D. Don) T. Moore, sed microsquamis usque ad 2 x 0.5 mm [(0.5-0.8) x (0.2-0.3) mm in P. obliquo], brunneis (brunneolis in P. obliquo), lamina basi non-contracta (leviter contracta in P. obliquo), paleis stipitis supra atrobrunneis (brunneis in P. obliquo), paleis rhachidis usque ad 3.6 x 0.8 mm (usque ad 2.3 x 0.5 mm in P. obliquo), pinnis atrovirentibus, subcoriaceis, supra nitidis, margine leviter repandis vel fere integris (pinnis viridibus, supra impolitis, margine serrulatis in P. obliquo) differt.
Plants perennial, evergreen, 5-14 cm tall. Rhizome 0.5-1 cm long, ascending; scales linear, brown, 0.2-3.6 mm long; roots dark brown when dry, up to 9 cm long, c. 0.6 mm in diam., sparsely or densely covered with scales. Leaves caespitose, 4-7 per rhizome; petiole 2-6 cm long, 0.6-1.2 mm in diam. at middle, adaxially canaliculate, green; basal petiole scales ovate-lanceolate, 3.6-4.5 x 1.1-2.3 mm, chartaceous, composed of multiple layers of cells, margin ciliate or erose, apex acuminate or caudate, brown, and matt, adaxially flat, dark brown, and lustrous; distal petiole scales ovate-lanceolate, 2.7-3.7 x 1.1-2.1 mm, differing in size, membranaceous, composed of 1 layer of cells, brown, margin regularly ciliate or with outgrowth, apex caudate, matt. Lamina lanceolate, 1-pinnate, 3.5-9.5 cm long, 1.2-2.6 cm wide at middle, 1.3-2.7 cm wide and broadest at base, apex acute; rachis 0.7-1.2 mm in diam. at middle, without proliferous buds, adaxially sulcate; scales of rachis similar to distal petiole scales but smaller, differing in size, margin regularly ciliate, apex caudate, matt. Pinnae 6-14 pairs, sparsely arranged, strongly reflexed toward lamina base, basal two pairs 0.7-1.5 cm apart, alternate, oblong, middle pinnae 7.5-12 x 3.5-5.5 mm, basalmost pinnae slightly larger, shortly petiolated, subcoriaceous, acroscopic base slightly auriculate, basiscopic base cuneate, forming 30-90-degree angle with rachis, apex acute, margin slightly repand-serrate ended in tiny tip or nearly entire and without aristate spinules, abaxially scaly, adaxially lustrous and glabrous; microscales on abaxial surface subulate with dilated base (broad-type microscales), 0.5-1.1 mm long, base 0.13-0.26 mm wide, with a few curly outgrowths on margin of base; venation pinnate; midrib slightly raised abaxially, flat adaxially; lateral veins free, 4-5 pairs from midrib per pinna, each lateral vein further dichotomous, indistinct on both surfaces. Sori terminal on veins of pinnae, 5-9 (1-3 below midrib, 4-6 above midrib) per fertile pinna, located approximately at middle between midrib and pinna margin and 0.9-1.6 mm distant from pinna margin; all pinnae on
|
|