|
|
|
|
|
|
|
|
|
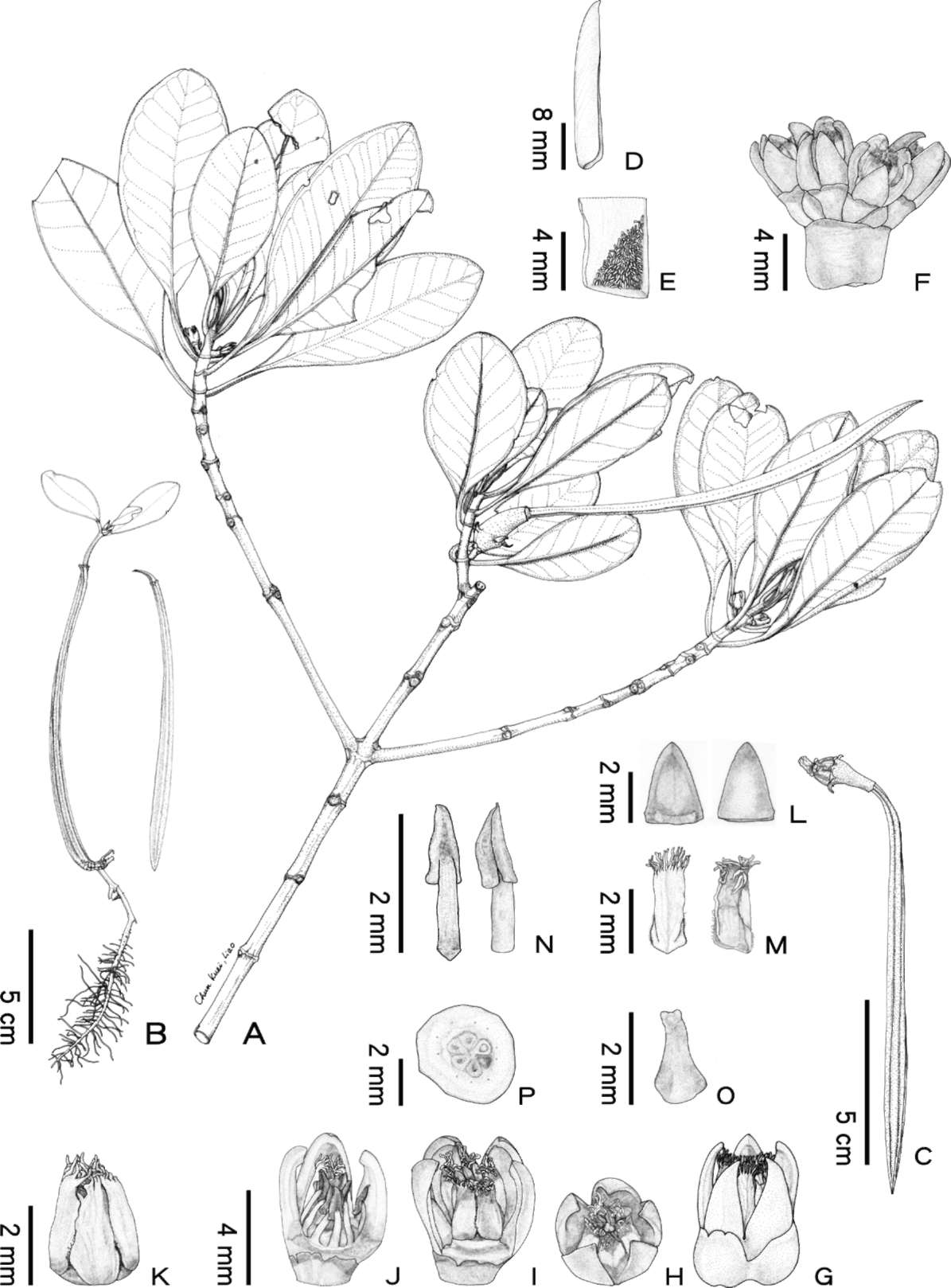
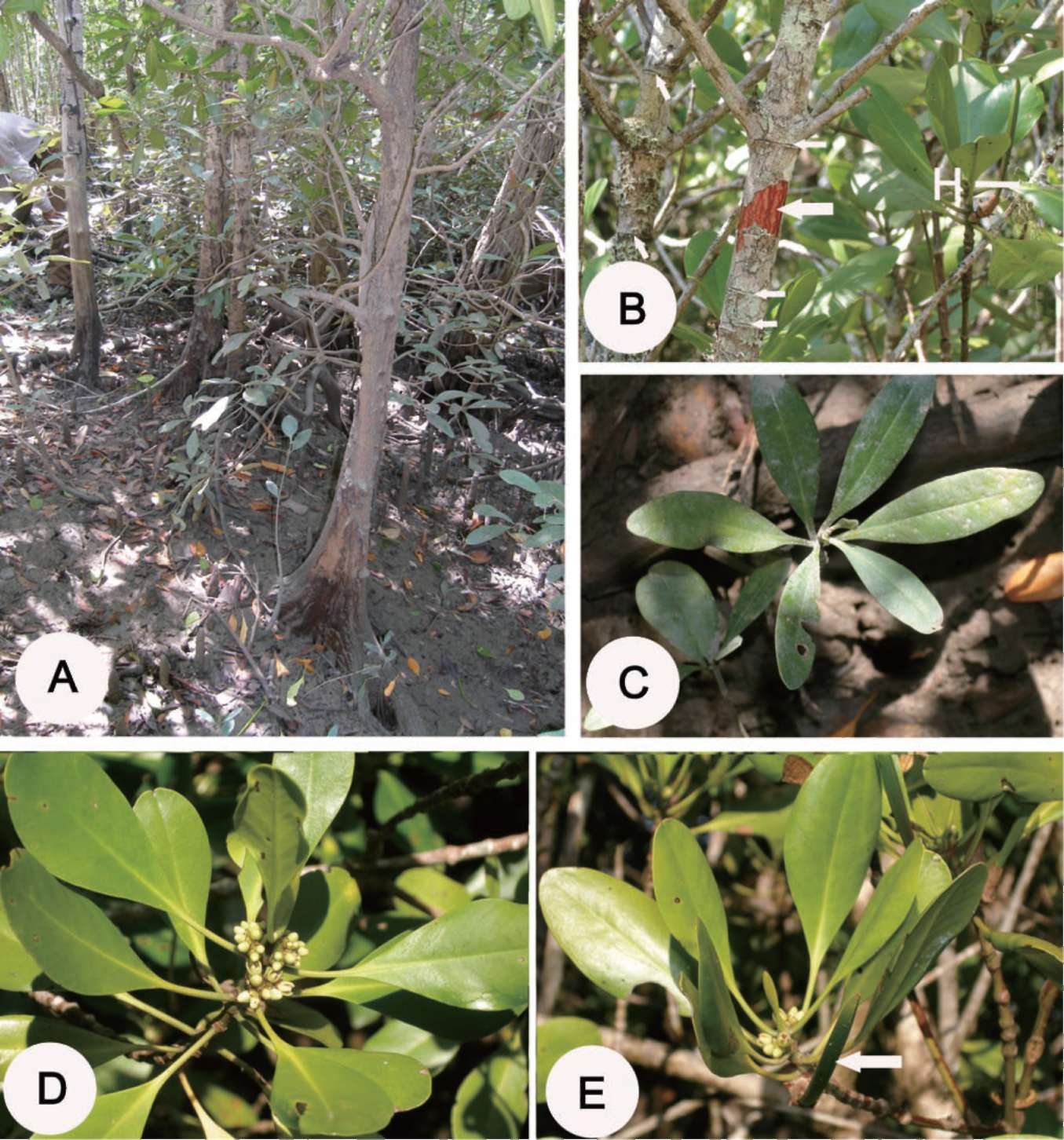
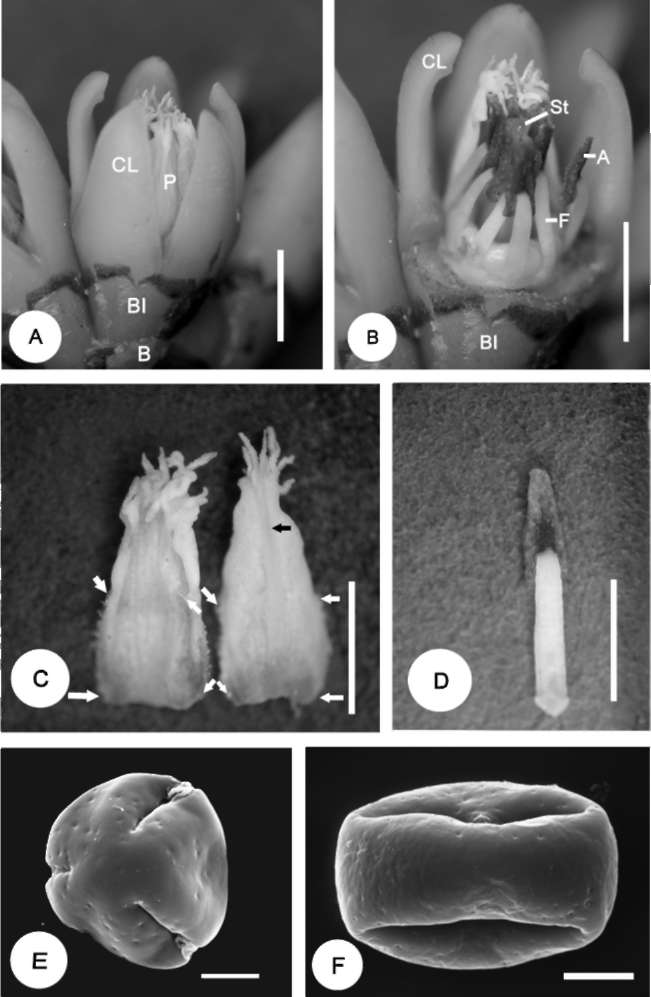
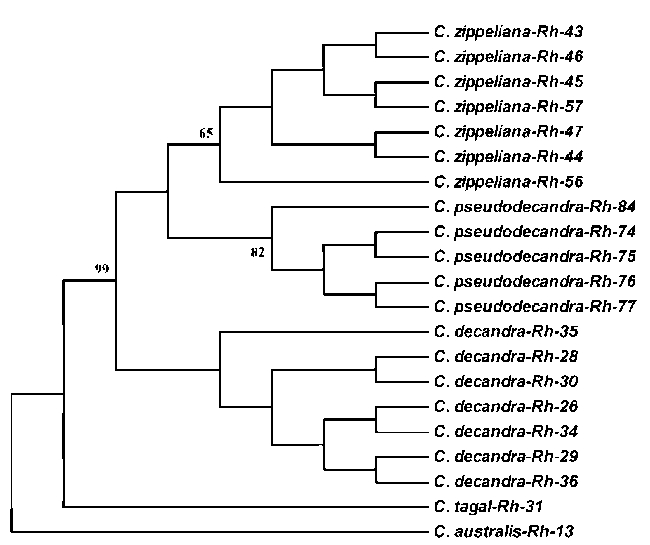
Table 2. The comparison of morphological characters among Ceriops decandra (Griff.) Ding Hou, C. pseudodecandra Sheue, Liu,
|
|
|
|
Tsai and Yang and C. zippeliana Blume.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.2-2.4 cm before dropping, with
|
2.0-3.0 cm before dropping, with 80-100 2.5-3.6 cm before dropping, with
|
|
|
|
|
50-70 colletors at adaxial base
|
colletors at adaxial base (8-12 layered)
|
154-190 colletors at adaxial base
|
|
|
|
|
|
|
|
|
|
|
|
Oval to obovate, 4.0-9.0 cm x
|
Oblong to elliptic-obovate,
|
Obovate-elliptic, 5.5-11.0 cm x 3.0-7.5
|
|
|
|
|
2.5-6.0 cm, lateral veins 8-10(-11),
|
6.0-12.0(-13.0) cm x 2.5-5.0(-6.0) cm,
|
cm, lateral veins (9-)11-12(-13),
|
|
|
|
|
petiole 1.2-1.8 cm in length
|
lateral veins 8-12, petiole 1.2-3.5 cm
|
petiole 1.5-2.6 cm in length
|
|
|
|
|
|
|
|
|
|
|
|
16 buds, (4-)6-10(-12) matured,
|
(2-)3-20 flowered, dense bifurcate
|
3-5(-7) flowered, simple head-like,
|
|
|
|
|
dense bifurcate cyme-like, with
|
cyme-like, with primary and additional
|
|
|
|
|
|
primary and additional bracts
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5 lobed, 2.0-3.0 mm x 2 mm
|
|
|
|
|
5, 4.0 mm x 1.8-2.0 mm (including
|
5, 2.0-2.5 mm x1.5 mm (including
|
5, 3.0-3.5 mm x1.8 mm (including
|
|
|
|
|
terminal cilia), entire margins
|
terminal cilia), upper half margins
|
terminal cilia), margins hairless, apex
|
|
|
|
|
with dense and long hairs (0.5
|
glabrous, lower half margins with
|
finger-like fringed with sinuate 13-17
|
|
|
|
|
mm), apex finger-like fringed with
|
loose and short hairs (< 0.1 mm), apex
|
|
|
|
|
|
20-25 sinuate cilia, 0.8-1.25 mm
|
finger-like fringed with 12-18 sinuate
|
|
|
|
|
|
|
|
|
|
|
|
|
10, filament 1.6-2.0 mm long,
|
10, filament 1.3 mm long, anther 0.9
|
10, filament 1.0 mm long, anther 1.0
|
|
|
|
|
anther 1.0-1.2 mm long, with one
|
mm long, with one short connective
|
mm long, with one short connective
|
|
|
|
|
long connective protrusion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
L = 21.0 士 1.49 jam in equator view,
|
L = 19.7 士 1.2 jam in equator view,
|
L = 15.43 ±1.16 jam in equator view,
|
|
|
|
|
exine scabrate with punctae
|
exine smooth with sparsely distributed
|
exine irregularly regulo-reticulate
|
|
|
|
|
|
|
|
|
|
|
|
Calyx hemi-globular (dome-like),
|
Calyx tube hemi-globular, 4-5 mm high,
|
Calyx tube shallow disc-like, 2-3 mm
|
|
|
|
|
5-9 mm high, persistent lobes
|
persistent lobes 5, 2.5-3.0 mm x 1.0
|
high, persistent lobes 5, 2-2.5 x 1-1.5
|
|
|
|
|
5, 4.0 x1.6-2.0 mm; fruit ovoid,
|
mm; fruit ovoid-conical, 1.0-1.3 cm x
|
mm; fruit ovoid-conical, 1.2-1.5 cm
|
|
|
|
|
0.6-1.0 cm x 0.5-0.6 cm, no
|
0.5-0.8 cm, no special decoration
|
x 1.0 cm, with netted decoration
|
|
|
|
|
|
|
|
|
|
|
|
8-13 cm x 0.5-0.7 cm, gradually
|
10-16 cm x 0.5-0.8 cm, gradually
|
9-17 cm x 0.7-0.8 cm, gradually
|
|
|
|
|
thickening with a blunt apex (root
|
thickening with an acuminate sharp
|
thickening with a acute sharp apex
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|