378
Botanical Studies, Vol. 51, 2010
may vary greatly among several areas of a given hotspot. For example, in the Mediterranean Basin hotspot, the population and livestock growth represents the main threat to the plant biodiversity on the southern shores whereas the northern part of the basin is affected by the expansion of woodlands (dominated by competitive species) in the inland areas and the habitat fragmentation in costal areas (Medail and Quezel, 1999). Thus, studies at a finer scale of resolution (i.e. defining hotspots within hotspots) are needed in order to design suitable conservation strategies at a more local level.
It is well-known that the tropics harbor a high proportion of global biodiversity (Pimm and Brown, 2004). The area from South China to North Vietnam harbors very high levels of biological diversity, but, at the same time, faces exceptional threats (Sodhi et al., 2004). Whereas the tropical areas of this region are included in one of the 25 global biodiversity hotspots (Indo-Burma; Myers et al., 2000) some of the northern parts (belonging to the southern subtropics) are recognized as world centers of plant diversity (CPDs; Davis et al., 1995). Furthermore, some of the major plant glacial refugia of China are included here (Wang and Liu, 1994; Lopez-Pujol, 2008), and the largest limestone areas in the world, together with their irreplaceable vegetation and flora, are also located in this Asian region (De Laubenfels, 1975; Yuang, 1993; Xu, 1995). However, the inventorying of the biological diversity in this region is largely insufficient. Hundreds of new taxa, including some new genera, have been described from there in recent years, especially from the limestone areas (e.g. Li and Wang, 2004; Tillich, 2005; Tillich et al., 2007; Peng et al., 2008). Meanwhile, neither the biodiversity distribution patterns nor its protection status within the area have been thoroughly evaluated. To address all these issues, the Guangxi Zhuang Autonomous Region (hereafter referred to as Guangxi Province), a region encompassing both tropical and subtropical areas, has been selected as a study case.
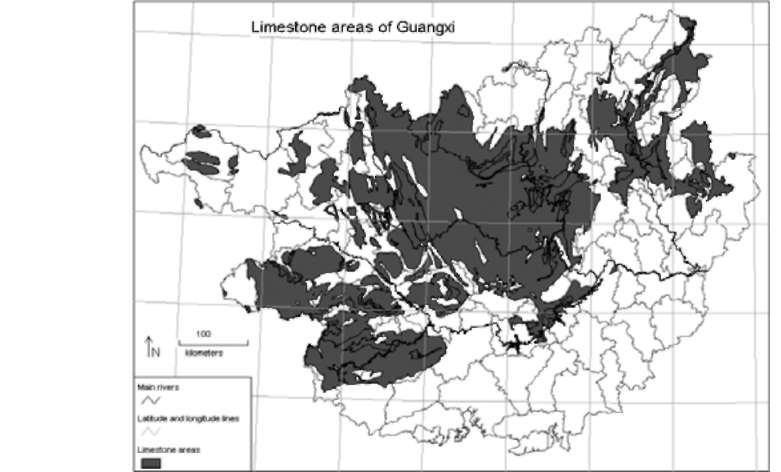
Guangxi Province lies between 20°54' to 26°23' N and 104°28' to 112°04' E in Southern China. It covers an area of ca. 236,000 km2, which is larger than many Mesoamerican and European countries. It stretches from the margin of the Karst Plateau in Southwest China in the west to seashore in the east with elevation decreasing from above 2,000 m to zero, and shares ca. 600km boundary with Vietnam (Figure 1). Limestone areas cover up to 40% areas of the province (Zhou et al., 2004; Figure 5), thus comprising it one of the largest limestone areas of the world when joined with limestone areas of neighboring provinces (mainly Guizhou and Yunnan provinces) and North Vietnam. A diverse flora (over 8,000 vascular plants) and very high levels of endemism (10 genera and 744 species of vascular plants endemic to the area) have been reported (Lu et al., 1989; Xu, 1995; Wu and Chen, 2004). Three regions of Guangxi, namely Nanling Mountain Ranges, Southern Guangxi Province, and the Limestone Region have been listed as world centers of plant diversity
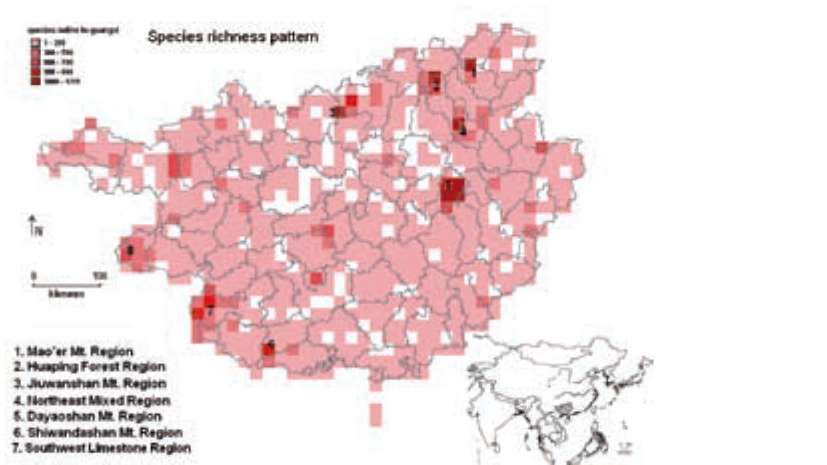
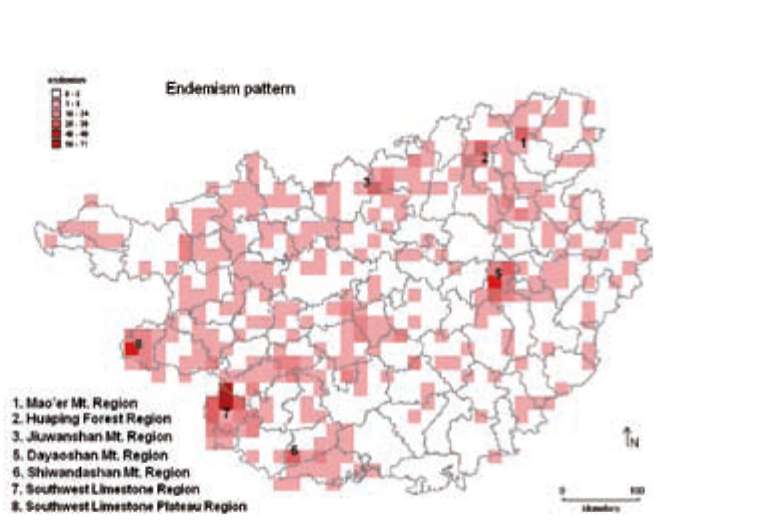
Figure 1. Species richness mapped in 10'x10' grid cells. Numbers in the map correspond to the centers of species richness. The letter 'c' indicates the location of Cenwanglaoshan Mountain.
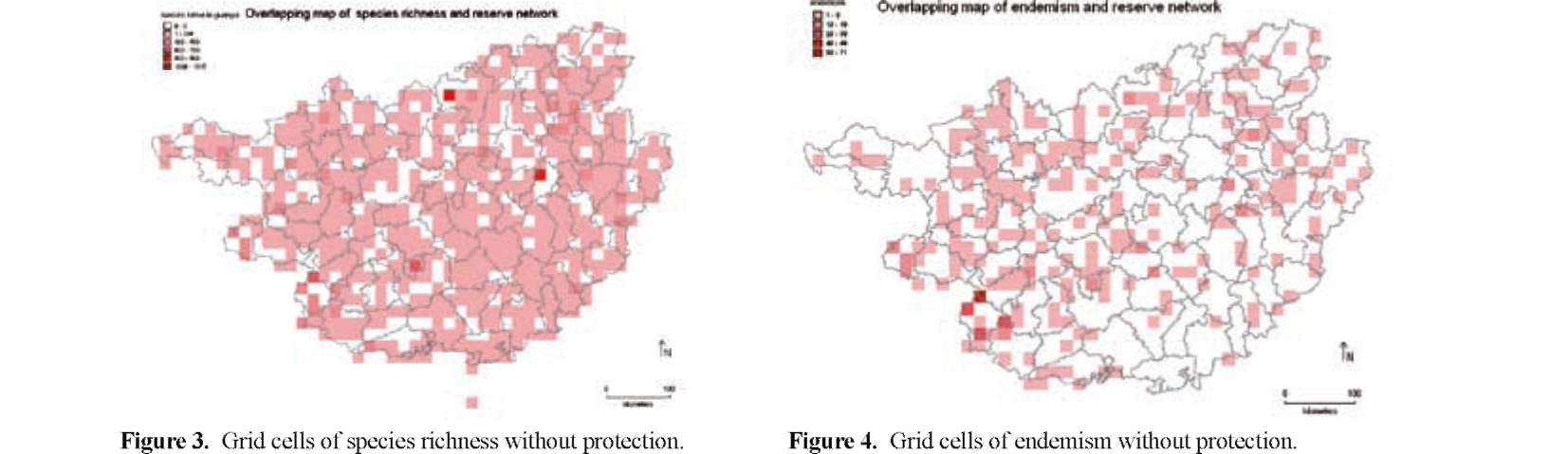
(CPDs) (Davis et al., 1995), and two more, Central Guangxi Mountains and West Guangxi Region have been identified as distribution centers by local botanists (Lu and Liang, 1983; Lu et al., 1989). The limestone region of the southwestern section of the province was recognized as one of the 11 "critical regions for biodiversity conservation in terrestrial China" (SEPA, 1998), and was included in the Indo-Burma world biodiversity hotspot (Myers et al., 2000), which is, in turn, one of the most threatened (Brooks et al., 2002; Sodhi et al., 2004). To protect the rich biodiversity, a nature reserve network including 72 reserves (which represents ca. 6% of the total land area) has been gradually established since 1961 (SEPA, 2007). However, no evaluation on their protecting efficiency has been done.
The main aim of this study is to identify plant diversity hotspots and to check their protection status in Guangxi, a region which represents the large conservation challenges to which the southern subtropical and northern tropical East Asia is currently facing. Herbarium collection records gathered from the region were used to map the distribution patterns of species richness and endemism. In addition, by comparing the obtained maps of plant biodiversity and the distribution of nature reserves, the protection efficiency of present reserve network can be envisaged.
materials and methods
Data collection and treatment
A database was compiled using collection records gathered from Guangxi Province. Thirteen main Chinese herbaria [CDBI, GXMI, HIB, HITBC, HNWP, IBK, IBSC, IFP, KUN, LBG, NAS, PE, WUK (the acronyms follow Fu, 1993)] were checked throughout from the Chinese Virtual Herbarium, a specimen information sharing platform in China (www.cvh.org.cn). The geographical