Botanical Studies (2010) 51: 403-411.

Diversity of waxy gene alleles in the wild rice species of
the Oryza genus
the Oryza genus
Zai-Quan CHENG1, Yan-Ping LIU1,2,# , Rui CHEN1, Bo PENG3, Hua-Bin XIONG4, Cheng ZHANG1,
Qiao-Fang ZHONG1, and Xing-Qi HUANG1,2, *
Qiao-Fang ZHONG1, and Xing-Qi HUANG1,2, *
1 Biotechnology & Genetic Germplasm Institute, Yunnan Academy of Agricultural ^Sciences, Kunming 650223, P.R. China
2 College of Life Science, Yunnan University, Kunming 650091, P.R. China
3Huazhong Agricultural University, Wuhan 430070, P.R. China
4Yunnan Ethnic University, Kunming 650091, P.R. China
3Huazhong Agricultural University, Wuhan 430070, P.R. China
4Yunnan Ethnic University, Kunming 650091, P.R. China
(Received October 22, 2008; Accepted April 1, 2010)
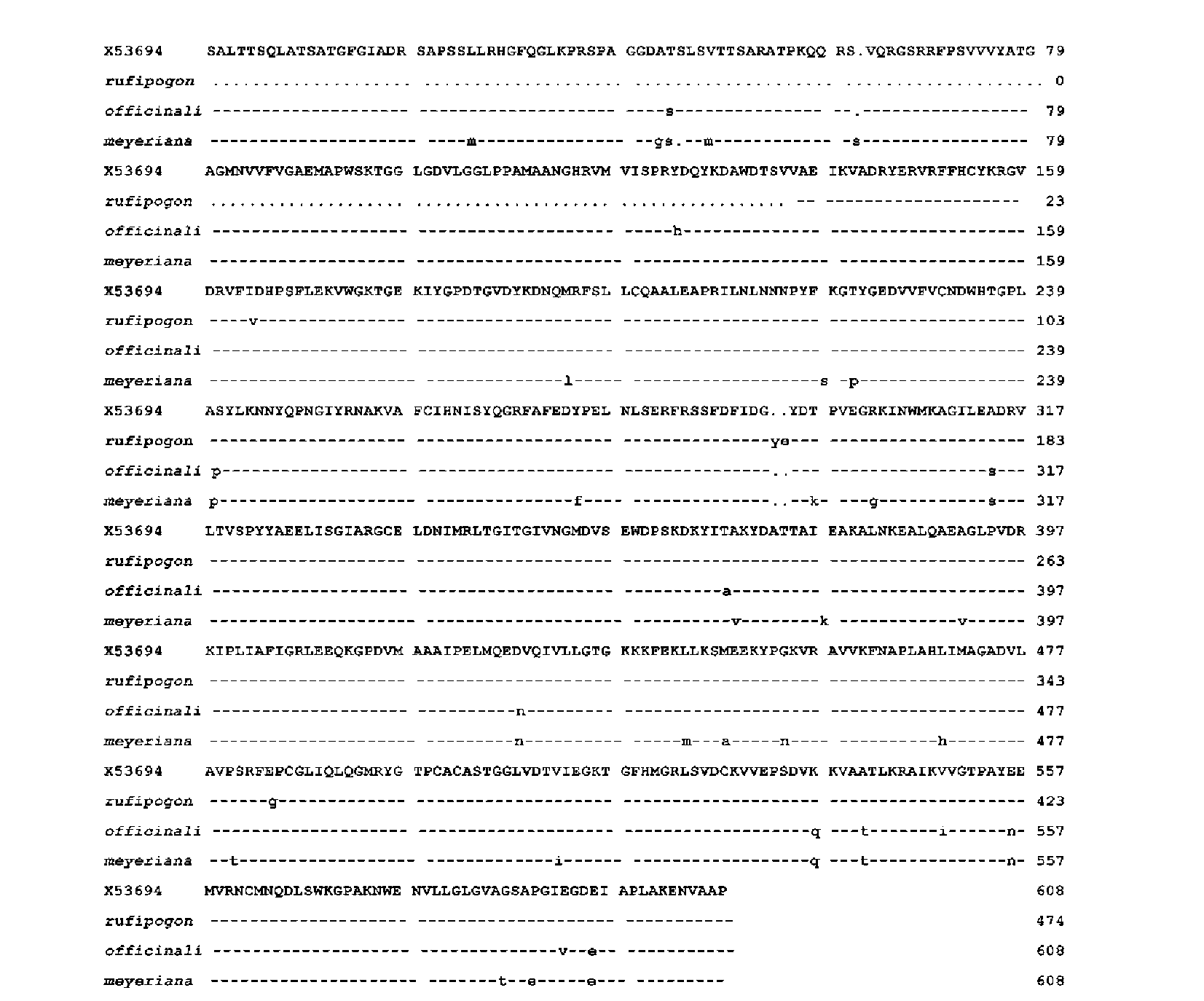
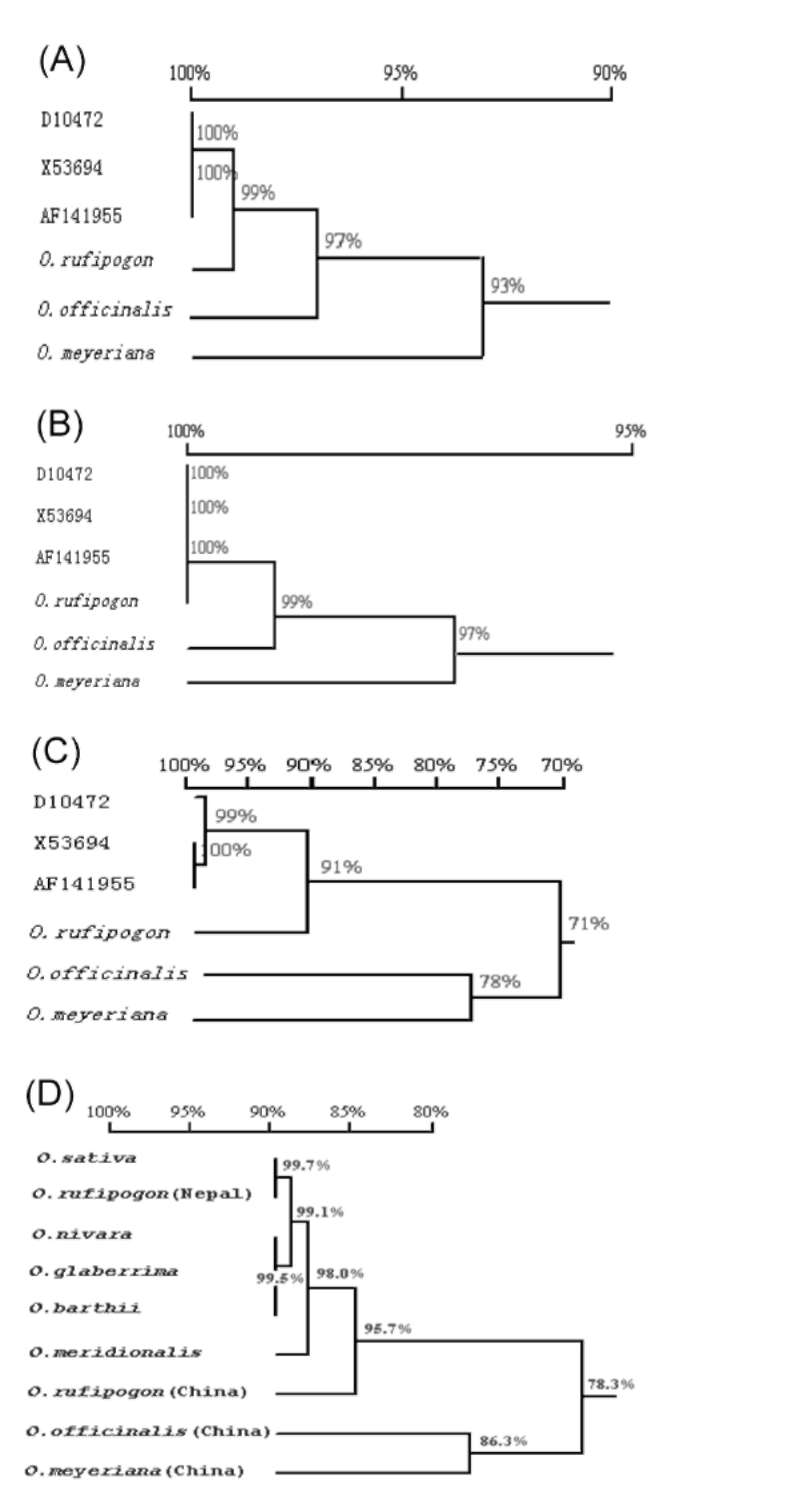
ABSTRACT. Amylose content and granule-bound starch synthase activity were measured for wild rice species (Oryza rufipogon, Oryza officinalis, Oryza meyeriana) and four widely cultivated rice varieties. The result indicates that the activity of GBSS in all rice species is positively correlated with the amylose content. The waxy gene alleles and their transcripts were cloned from three wild rice species using PCR amplification from genomic DNA or RT-PCR amplification from mRNAs of immature seeds. The waxy gene alleles of the three wild rice species and four cultivated rice varieties had different genomic DNA sequence sizes, intron-exon structures, and amino acid sequences. The predicted secondary structures of waxy proteins from the wild rice and cultivated species differed significantly. The waxy allele of O. rufipogon was most closely related to those of the cultivated rice varieties, with O. meyeriana less closely related and O. officinalis most distantly related. All of these results suggest that the allelic diversity of the waxy gene in Oryza genus is very rich. There might be different regulation mechanisms controlling amylose content by waxy genes in the wild rice species compared with the cultivated rice varieties (Oryza sativa L.).
Keyword:
Cloning and analysis; Gbss enzyme; Waxy gene allele; Wild rice.
INTRODUCTION
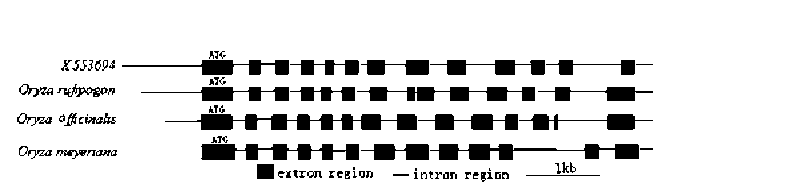
A few waxy gene alleles in different cultivated rice varieties (Oryza sativa L. AA genome 2n=24) with high (25-30%) or medium amylose contents (15-20%) have been reported (Cai et al., 2000). It is found that in general, the medium amylose contents in some cultivated rice varieties are mainly caused by the G to T change at the 5' splice junction of the first intron in the waxxy genes, which alters GBSS activity (Cai et al., 2000). The G to T point mutation in the first intron of the waxy gene alleles interrupts the formation of mature mRNA which causes low synthesis of amylose in the cultivated rice varieties studied (Zhu et al., 2004a; Zhu et al., 2004b; Li et al., 2005). The other introns and exons of the waxy genes were found to be quite conservative in the cultivated rice varieties of O. sativa (Zhu et al., 2004a,b; Bligh, 1995). These previous studies have showed that, the genetic diversity of the waxy genes of O. sativa rice varieties is not rich (Frances et al., 1998). Whether other Oryza species including some wild rice species also have conservative DNA sequences of waxy alleles is unknown.
Three wild rice species―Oryza rufipogon Griff (A'A' genome, 2n=24), the Oryza officinalis Wall (CC genome, 2n=24), and the Oryza meyeriana Baill (GG genome, 2n=24)―originating in China (Zhong and Cheng, 2000) were recently found to have relatively lower amylose content (10-14%) compared with most of the cultivated rice
Rice is one of the most important crops in the world. It is a staple food for more than half of the world's population. The demand for rice with better quality has been growing in the global market (Tang et al., 1989). The challenge now is to produce high quality cultivated varieties of O. sativa to satisfy this demand. Amylose content and proportion are important standards in appraising rice quality (Tan and Corke, 2002; Lim et al., 1995). Low and intermediate amylose content is considered an important factor that good quality rice should have in China and many other Asian countries like Japan and Korea. Granule-bound starch synthase (GBSS), an enzyme encoded by the waxy gene and responsible for the synthesis of amylose in rice endosperm (Denyer et al., 2001; Nakamura and Yuki, 1992; Nakamura et al., 1989) has been investigated in cultivated crops such as O. sativa indica or japonica rice varieties (Li et al., 2001; Ahmadi and Baker, 2001; Hirano and Yoshio, 1991; Geng et al., 2005; Cai et al., 2000), wheat (Kumar and Simgh, 1980), corn (Doehlert, 1993), and potato, but not in wild rice species.
#Co-first author.
*Corresponding author: E-mail: xingqih@public.km.yn.cn czquan-99@163.com; Tel: 86-871-5130681; Fax: 86-871-5160084.