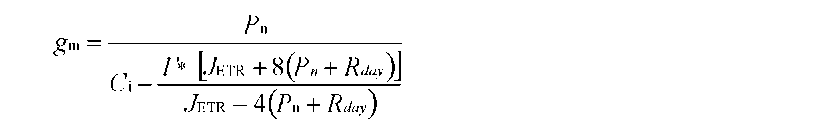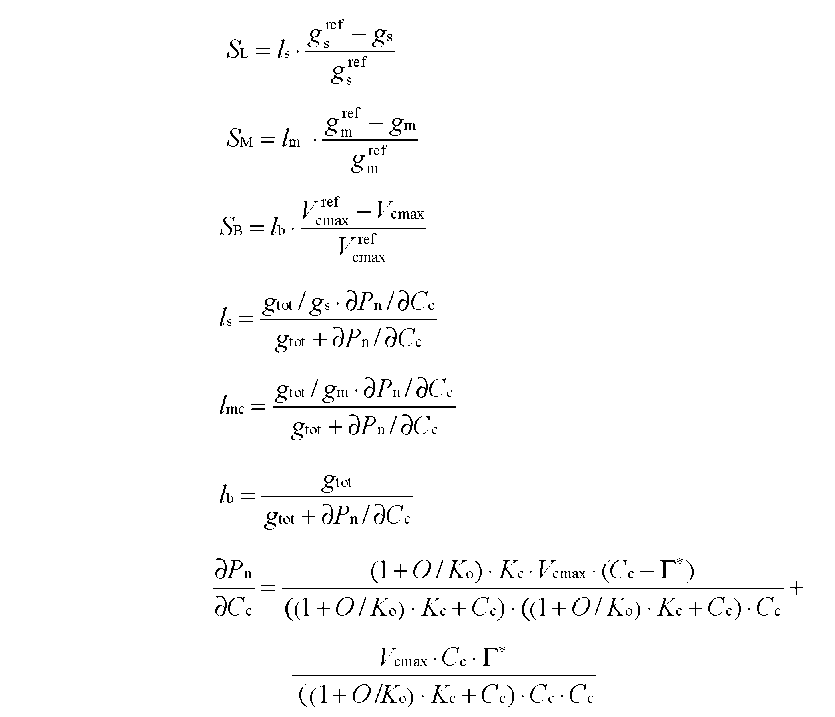Botanical Studies (2010) 51: 457-464.

Temperature acclimation of photosynthesis in Meconopsis horridula var. racemosa Prain.
Shi-Bao ZHANG
Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences,
Yunnan 650223, P.R. China
Yunnan 650223, P.R. China
(Received May 1, 2009; Accepted May 26, 2010)
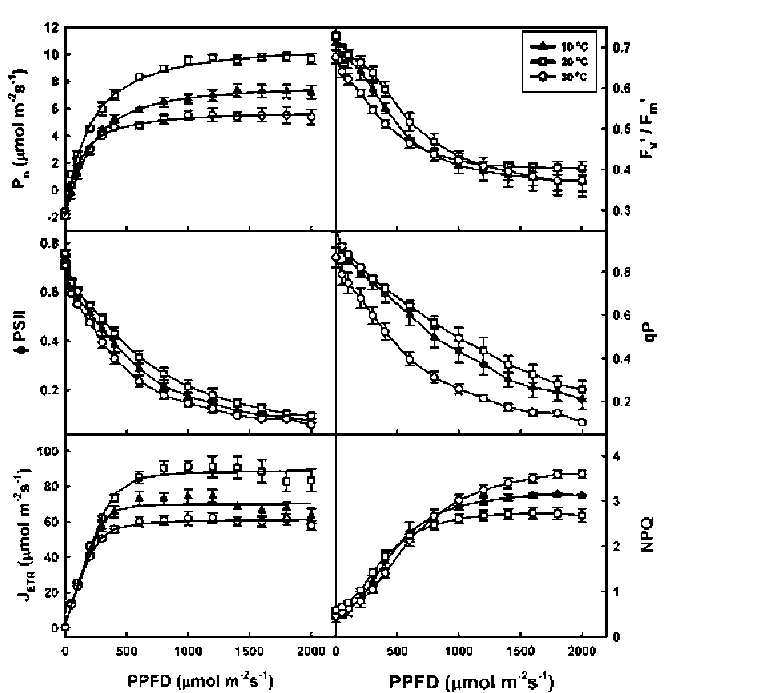
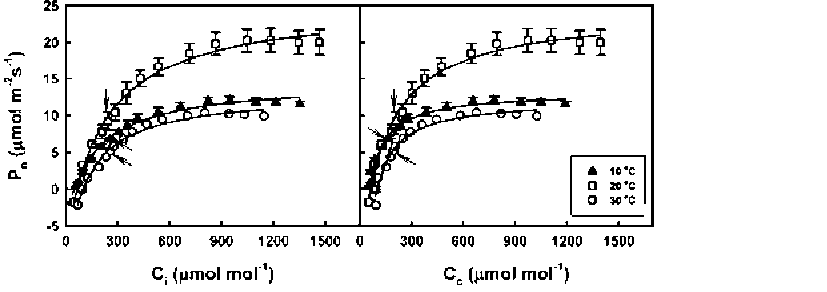
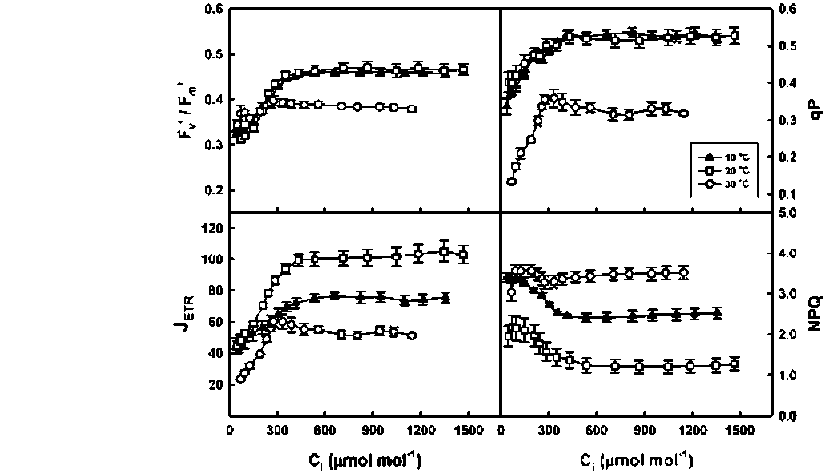
ABSTRACT. Meconopsis horridula var. race-mesa Prain. is a famous alpine flower and medicinal plant native to high elevations in the Himalayas, but cultivating it at lower altitudes presents great challenges. The photosynthetic gas exchange and chlorophyll fluorescence of M. horridula were investigated at three temperatures to evaluate its photosynthetic performance and the relative importance of biochemical limitation, sto-matal limitation, and mesophyll limitation to photosynthesis under different temperature regimes. Meconopsis horridula grown at 20°C could obtain the highest photosynthetic rate and photochemical efficiency among the three temperatures, and photosynthetic performance at low temperature was better than at high temperature. Non-photochemical quenching was an important mechanism protecting the photosynthetic apparatus of M. horridula under temperature stress conditions. Although mesophyll conductance was the dominant factor for limiting photosynthesis of M. horridula both at low temperature and high temperature, the photosynthesis at high temperature was also limited by stomatal conductance and biochemical efficiency. The poor photosyn-thetic performance at high temperature may be what limits M. horridula cultivation at low altitude.
Keywords: Chlorophyll fluorescence; High temperature; Meconopsis horridula; Photosynthesis; Photosyn-thetic limitation.
INTRODUCTION
remarkable tolerance to low temperature may result in the poor adaptability to high temperature. The growth and survival of plants can be limited by the thermo-tolerance capabilities of photosynthesis as photosynthetic traits govern carbon acquisition (Sharkey, 2000).
Several mechanisms for thermal acclimation of photosynthesis have been proposed. Plants grown at low temperatures have higher levels of Rubisco and other enzymes, which are involved in carbon metabolism compared with plants grown at high temperatures. Growth at low temperatures also results in higher levels of cytosolic fructose-1,6-bisphosphatase and sucrose-phosphate synthase, which regenerates orthophosphate during sucrose synthesis (Strand et al., 1999; Hikosaka et al., 2006). On other hand, high temperatures have been shown to alter thylakoid membrane structure, decrease Rubisco activity and RuBP regeneration capacity, perturb photosynthetic electron transport, increase dark respiration and photorespiration, and sequentially affect carbon assimilation (Yamasaki et al., 2002; Streb et al., 2003; Wise et al., 2004). However, the photosynthetic protective mechanisms vary greatly between plant species and ecotypes (Yamasaki et al., 2002). Unfortunately, little is known about the photosynthetic adaptation of Meconopsis to temperature. Such knowledge is particularly relevant to the domestication of wild species, the purpose of which is to protect wild populations from over-harvesting.
The majority of the 49 species in the genus Meconopsis belonging to Papaveraceae grow at high elevations (2,100-5,780 m) in the Himalayas and other mountains in western China. Only M. cambrica can be found in Europe (Chuang, 1981). As famous horticultural plants bearing beautiful flowers, Meconopsis has attracted the attention of botanists. Some Meconopsis species are also used as traditional herbal medicine for their anti-inflammatory and analgesic activities (Samant et al., 2005). The gene resources of Meconopsis have been increasingly threatened due to habitat destruction and over-harvesting of the plants from the natural habitats (Sulaiman and Babu, 1996). Several members of Meconopsis have been cultivated for over 100 years, but cultivating Meconopsis is not an easy task (Still et al., 2003).
Empirical observation suggests that high temperature during the growing season is an important determinant limiting the growth and development of Meconopsis (Norton and Qu, 1987; Ren, 1993). Both M. punicea and M. betonicifolia grown at 7.2°C /10°C (night/day temperature) have larger dry weight and flower size than at 18.3°C /21.1°C (Still et al., 2003). Meconopsis integrifolia can flower in its native habitat with snow on the ground. This
*Corresponding author: E-mail: zhangsb@xtbg.org.cn; Tel: 86-0871-5142069.
*Corresponding author: E-mail: zhangsb@xtbg.org.cn; Tel: 86-0871-5142069.