Botanical Studies (2010) 51: 465-472.

Impact of ultra-dry storage on vigor capacity and antioxidant enzyme activities in seed of Ammopiptanthus mongolica
Yi LI1'2'*, Jian-Jun QU1,2, Wei-Min ZHANG1,2, Li-Zhe AN3, Peng XU4, and Yong-Cai LI5
Chinese Academy of Sciences, Lanzhou Gansu, 730000, P.R. China
1Dunhuang Gobi and Desert Research Station, Cold and Arid Region Environmental and Engineering Research Institute,
Chinese Academy of Sciences, Lanzhou Gansu, 730000, P.R. China
2Key Laboratory of Desert and Desertification, Chinese Academy of Sciences, Lanzhou Gansu, 730000, P.R. China
3School of Life Science, Lanzhou University, Lanzhou Gansu, 730000, P.R. China
4Pratacultural College, Gansu Agricultural University, Lanzhou Gansu, 730070, P.R. China
5College of Food Science and Engineering, Gansu Agricultural University, Lanzhou Gansu, 730070, P.R. China
(Received July 7, 2009; Accepted May 14, 2010)
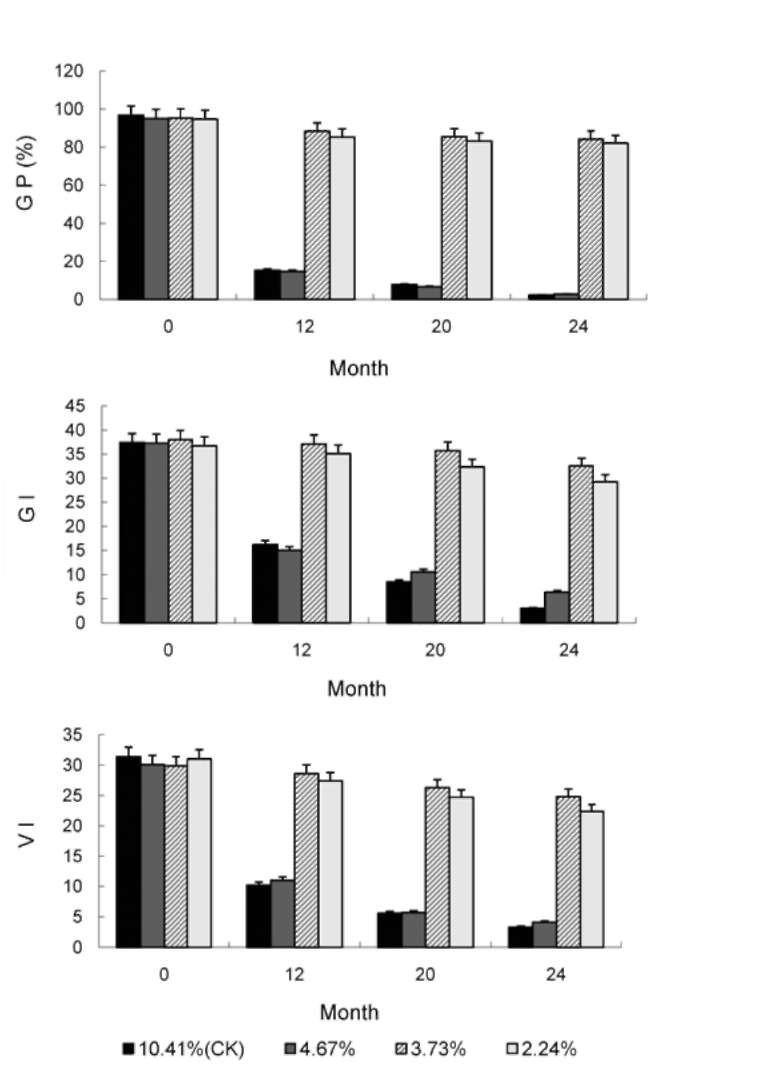
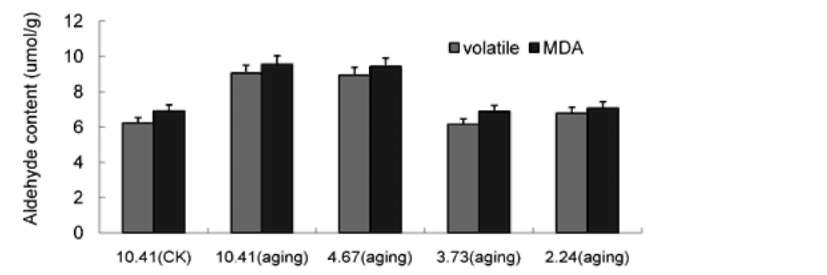
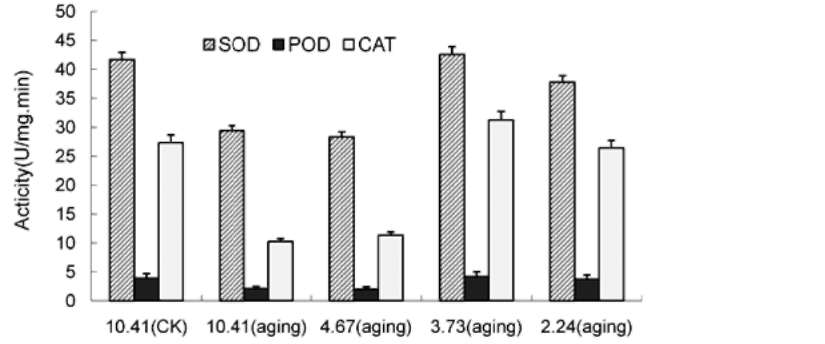
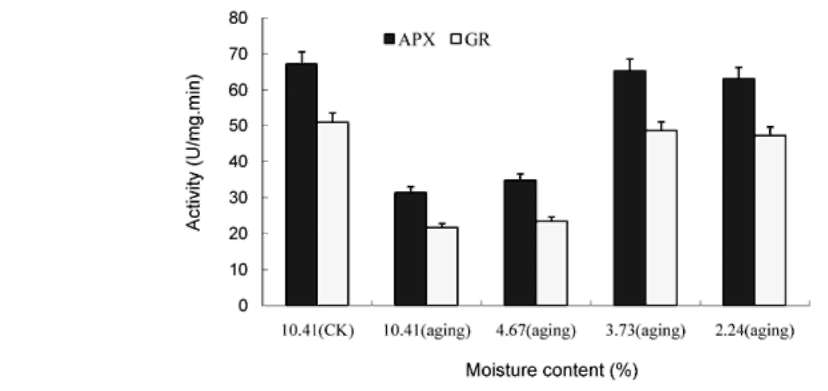
ABSTRACT. This research was to determine whether ultra-drying improves the vigor and storability of Ammopiptanthus mongolica seeds. Seeds of A. mongolica were dried to a 4.67%, 3.73%, and 2.24% moisture content (MC). After storage for 24 months, their level of vigor was measured. To determine whether these low MCs affect the activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR), we evaluated the ability of dried seeds to germinate and to produce normal seedlings. Meanwhile, we also measured volatile aldehydes and malondial-dehyde (MDA), lipid peroxidation production. Results indicated that the SOD, CAT, POD, APX and GR activities of ultra-dry seeds were higher than those of control seeds while volatile aldehydes and MDA were lower than in the control. The drying tolerance was related to a reorientation of the enzymatic antioxidant defence system. The electrical conductivity of ultra-dry seeds and controls showed obvious differences. All the results showed that ultra-dry storage is beneficial to maintaining the vigor of A. mongolica seeds. It is proposed that A. mongolica seeds may be stored in an ultra-dry state at ambient temperature for a long time.
Keywords: Antioxidant defence system; Seed longevity; Seed storability.
INTRODUCTION
Desiccation is that part of a seed's life programme that permits its storage and survival in various environment conditions (Leprince et al., 1993). The ability of seeds to withstand severe desiccation generally occurs during the phase of reserve accumulation, but this is dependent on the drying rate, which has been shown to affect seed survival after drying (Kermode, 1995; Pammenter and Berjak, 1999; Bailly et al., 2001). Many cellular and biochemical events appear associated with the desiccation tolerance of seeds. They include events that modify ultrastructural characteristics like vacuolation, synthesis of dehydrins, heat shock proteins, and activation of antioxidative defences (Galau et al., 1991; Vierling, 1991; Leprince et al., 1993; Vertucci and Farrant, 1995; Folkert et al., 2001, Bailly et al., 2001). Reactive oxygen species (ROS) are of increasing interest in seed physiology. Most contributions to date in this field have been concerned with the role of
*Corresponding author: E-mail: liyi2001@gmail.com.
*Corresponding author: E-mail: liyi2001@gmail.com.
ROS in loss of vigor and viability during the storage of seeds (Bailly et al., 2004). Lipid peroxidation induced by ROS has been widely cited as a major cause of seed aging (Priestley, 1986; Mcdonald, 1999). Dehydration of seeds has been associated with the impairment of antioxidative mechanisms leading to oxidative damage and to numerous lethal lesions (Pammenter and Berjak, 1999; Li and Sun, 1999; Bailly et al., 2004). Tolerance to drought of vegetative tissues is thought to be related to antioxidant enzymes (Sherwin and Farrant, 1998). However, data on the role of antioxidant systems during the seed desiccation tolerance is lacking. The ability of seeds to bear desiccation could be associated with their ability to scavenge ROS in order to avoid deleterious events such as lipid peroxidation. These mechanisms might involve enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR) (Bailly et al., 2001). However, seed germinability might be related to the efficiency of free radical scavenging because this scavenging may affect merely seed storability and vigor (Priestley, 1986; Bailly et al., 2000;
Bailly et al., 2001).