|
|
|
|
|
|
|
|
|
|
|
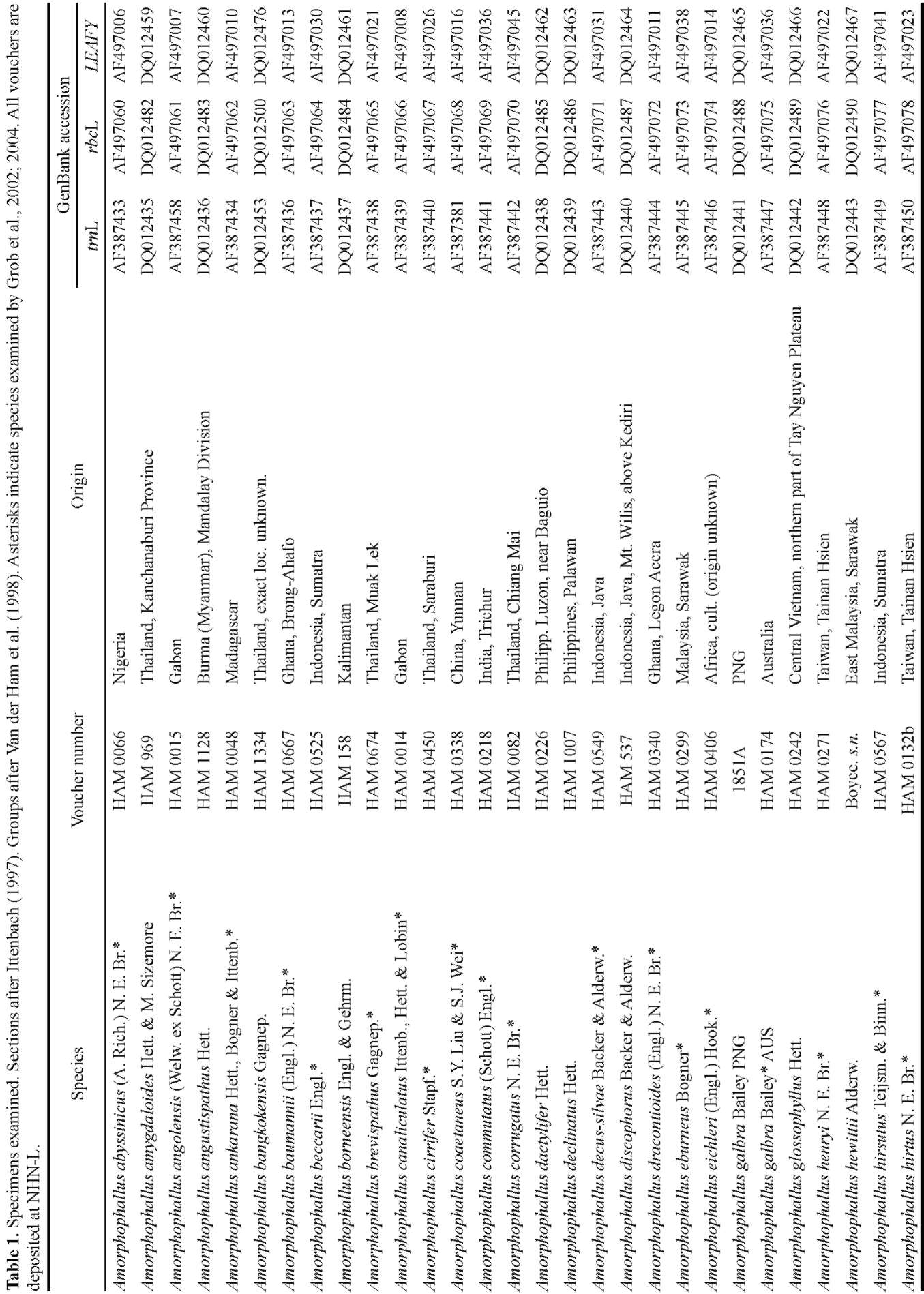
Botanical Studies (2010) 51: 473-490.
|
|
|
|
|
|
|
|
|
Morphological character evolution of Amorphophallus
(Araceae) based on a combined phylogenetic analysis of
trnL, rbcL and LEAFY second intron sequences
|
|
|
|
|
|
|
|
Agung SEDAYU2, Marcel C. M. EURLINGS3, Barbara GRAVENDEEL3 and Wilbert L. A.
HETTERSCHEID1*
|
|
|
|
|
|
|
|
1 National Herbarium of the Netherlands, Wageningen University Branch, Wageningen University Botanical Gardens (presently: Von Gimborn Arboretum, Doorn, Netherlands)
2 Bogor Botanical Gardens, Indonesia
3 Netherlands Centre for Biodiversity Naturalis, National Herbarium of The Netherlands, Leiden University
|
|
|
|
|
|
|
|
(Received January 24, 2009; Accepted April 21, 2010)
|
|
|
|
|
|
|
|
ABSTRACT. Sequences of three different genes in 69 taxa of Amorphophallus were combined to reconstruct the molecular phylogeny of this species-rich Aroid genus. The data set was analyzed by three different methods, Maximum Parsimony, Maximum Likelihood and Bayesian analysis, producing slightly different tree topologies. Three major clades identified in all analyses reflect the biogeographical distribution of Amorphophallus. Some clades were supported by morphological characters such as sessile/nonsessile stigma, pollen opening mechanism, shape of the main segments of the lamina, growth cycle, and berry colour. When optimised, a nonsessile stigma may have evolved from a sessile one with several reversals. Pollen opening by connective rupturing evolved from pollen opening by pores. Unequally shaped segments of the lamina evolved from equally shaped segments. Simultaneously existing leaf and inflorescences evolved from alternating leaves and inflorescences. Blue, purple, green, and yellow berries evolved from red/orange/white ones.
|
|
|
|
|
|
|
|
Keywords: Amorphophallus; Araceae; Character optimization; LEAFY; Molecular phylogeny; rbcL; trnL.
|
|
|
|
|
|
|
|
INTRODUCTION
The species-rich Aroid genus Amorphophallus currently encompasses ca. 200 species distributed throughout the (paleo)tropics, from West Africa, the western border, eastward into Polynesia and southeastward to Australia (1 species). The eastern distribution border is due to the cultivated species A. paeoniifolius, which might not represent a natural distributional border. The northernmost border of the genus is situated in the tropical and subtropical areas of Central Asia, China, and South Japan (Hetterscheid and Ittenbach, 1996). A recent molecular study using a combination of matK and rbcL sequences revealed the position of Amorphophallus and other Araceae as a monophyletic clade in a basal node of the order Alismatales (Tamura et al., 2004). Araceae, together with Arecaceae and Orchidaceae, were also found among the oldest families of monocot with crown node ages reaching back into the Early Cretaceous (Wikstrom et al., 2001; Jannsen and Bremer, 2004). The most recent study (Cabrera et al., 2008) of Araceae phylogeny, using a combination of matK, rbcL, the trnK intron, the trnL intron, and the trnL-trn¥ spacer, shows the tribe
*Corresponding author: E-mail: hetter@xs4all.nl.
|
Thomsoniae (Amorphophallus + Pseudodracontium) as a basal sister-clade to a clade consisting of the tribes Caladieae and Zomicarpeae.
Amorphophallus was first placed in the tribe Thomsoniae (Blume, 1835; Bogner et al., 1985). Tribe Thomsoniae consisted of two closely related genera, Amorphophallus and Pseudodracontium. Molecular evidence indicates that these two genera could be merged into a single genus, Amorphophallus (Grob et al., 2002, 2004).
Several attempts have been made to reveal the phylogenetic relationships within the genus Amorphophallus sensu lato (incl. Pseudodracontium). The latest studies (Grob et al., 2002, 2004) used molecular data for phylogenetic reconstruction. This paper attempts to interpret morphological character evolution in Amorphophallus based on a combined nuclear and plastid phylogeny that is more completely sampled than in previous studies. The species sampled were carefully selected to come up with a representative subset of the total morphological diversity present in Amorphophallus. The morphological characters by which these species differ were coded (for more details, see below) and then plotted on the most likely molecular phylogenetic tree reconstructed to trace their evolution.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
|
thermal cycling protocol consisted of 35 cycles with an initial denaturation phase at 94°C for 5 min, followed by a denaturation at 94°C for 30 s, annealing at 52°C for 30 s, an extension phase at 72°C for 1 min and a final extension of 5 min at 72°C. The PCR products were purified using QIAquick PCR purification columns (Qiagen) and eluded in 50 fiL elution buffer. Cycle sequences for all three genes were performed using identical primers at a lower concentration (2.5 fM). Sequence products were cleaned by Sephadex G50 AutoSeq columns (Amersham-Pharmacia Biotech) and run on an ABI 377 Prism Automatic sequencer (PE Applied Biosystems).
|
|
|
|
|
|
|
|
Of a total of 25 species of Amorphophallus, the rbcL gene, trnL intron, and LEAFY second intron were se-quenced. These markers were chosen because they provided sufficient phylogenetic resolution in previous studies (Grob et al., 2002, 2004). Frohlich and Meyerowitz (1997) also pointed out that the second intron of LEAFY might have evolved at a high rate and might be useful for reconstructing phylogenies of closely related species. LEAFY was also shown to provide more phylogenetically informative characters than nrITS, trnL-trnF, trnD-trnT, or matK-trnK by Oh and Potter (2003).
The sequences obtained were then combined with sequences of another 44 species of Amorphophallus, two of Pseudodracontium and one outgroup already previously published. Among the 25 species sequenced in this study, A. bangkokensis (= A. paeoniifolius var. bangkokensis) and A. galbra from Papua New Guinea were sampled to compare them with A. paeoniifolius and A. galbra from Australia already previously sequenced. Hapaline sp. representing Caladieae was chosen as an outgroup because of its close relationship with Amorphophallus (French et al., 1995; Cho and Palmer, 1999; Rothwell et al., 2004).
Total genomic DNA was obtained from fresh leaf tips of living specimens at the Hortus Botanicus Leiden and Wageningen University Botanical Garden using the DNeasy Plant Mini Kit (Qiagen) following the manufacturer's protocol. DNA sequences obtained were deposited in GenBank (Table 1).
|
|
|
|
|
|
|
|
Raw sequences were examined, translated and corrected using Sequencher 4.0.1 (1998). Corrected sequences were directly aligned by eye using PAUP* 4.0b10 for Microsoft Windows and MacClade version 4.06 (Maddison and Maddison, 2003). When ambiguous bases were encountered, chromatograms were checked. BLAST searches were done to confirm gene identity. Shared indels were treated as single characters. Insertions were coded as 1 and the deletions as 0. Inapplicable character states (such as appendix diameter in case of an absent appendix) were coded as missing.
|
|
|
|
|
|
|
|
Maximum Parsimony (MP) trees were reconstructed with PAUP* using unweighted maximum parsimony. Heuristic searches were performed with 100 replicates, random addition, and TBR swapping. Three different heuristic searches were performed, each with different MaxTree settings (10,000; 25,000; infinite). The tree file from the infinite MaxTree setting was used to compute Bootstrap Support (BS) values. Bootstrap analysis was performed using 2000 replicates, each with 100 heuristic searches with random additional sequences, TBR swapping and 10 trees saved per replicate. Nodes with over 70% BS were considered significantly supported (Soltis and Soltis, 2003). Maximum Likelihood (ML) analyses were carried out using PAUP* to calculate the tree with the highest likelihood score which was subsequently used for morphological character optimization. Bayes-ian analysis was done using MrBayes 3.0 (Ronquist and Huelsenbeck, 2003). Nucleotide substitution models were determined separately for each gene using MrModeltest 1.1b (Nylander, 2002) to determine the best substitution model. The Bayesian analysis was initiated with random starting trees and run for 2 x 106 generations. One tree was saved every 10 generations. After 250,000 generations, a stable probability was reached. All non-significant generations (p < 0.5) were discarded for the consensus tree.
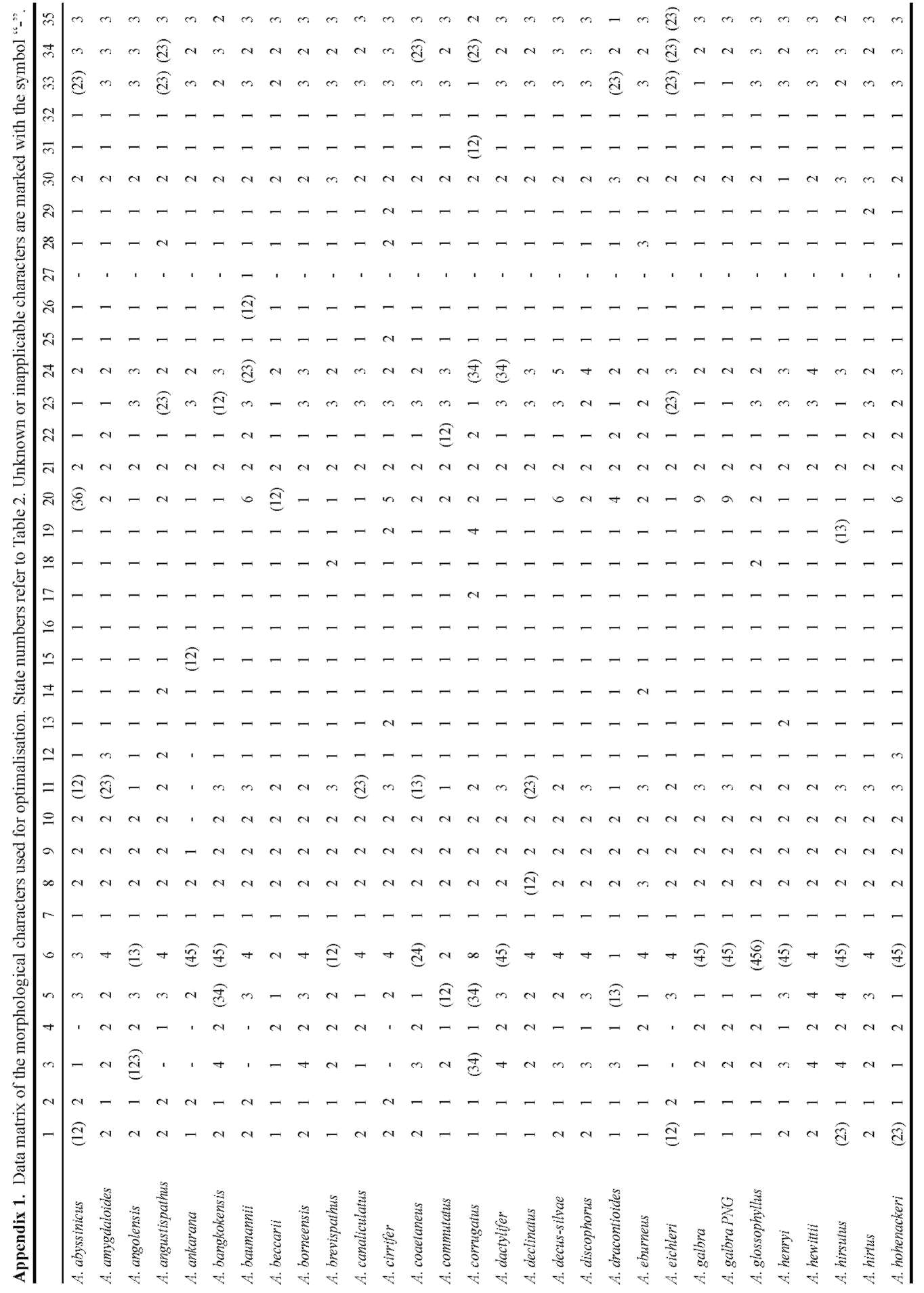
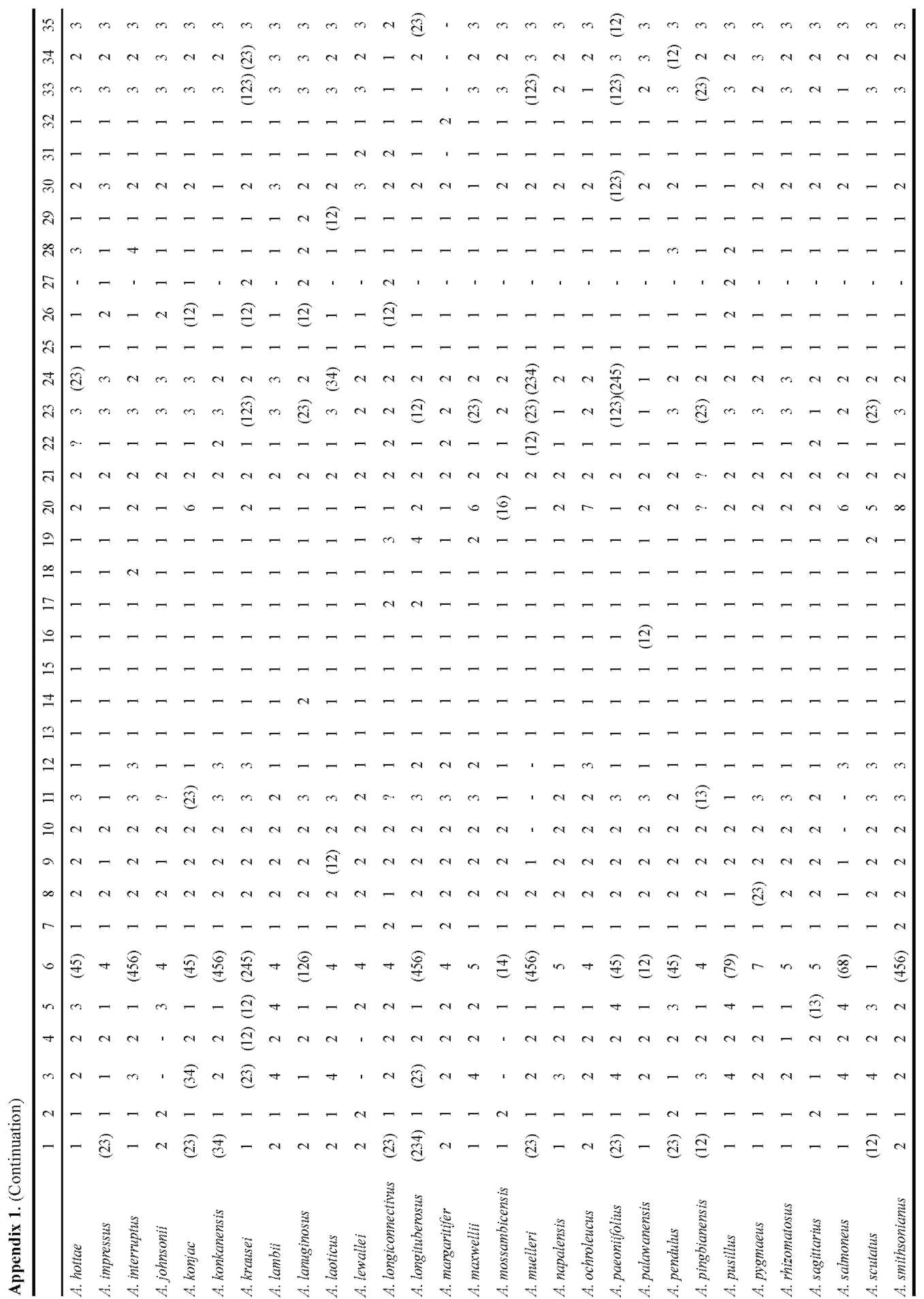
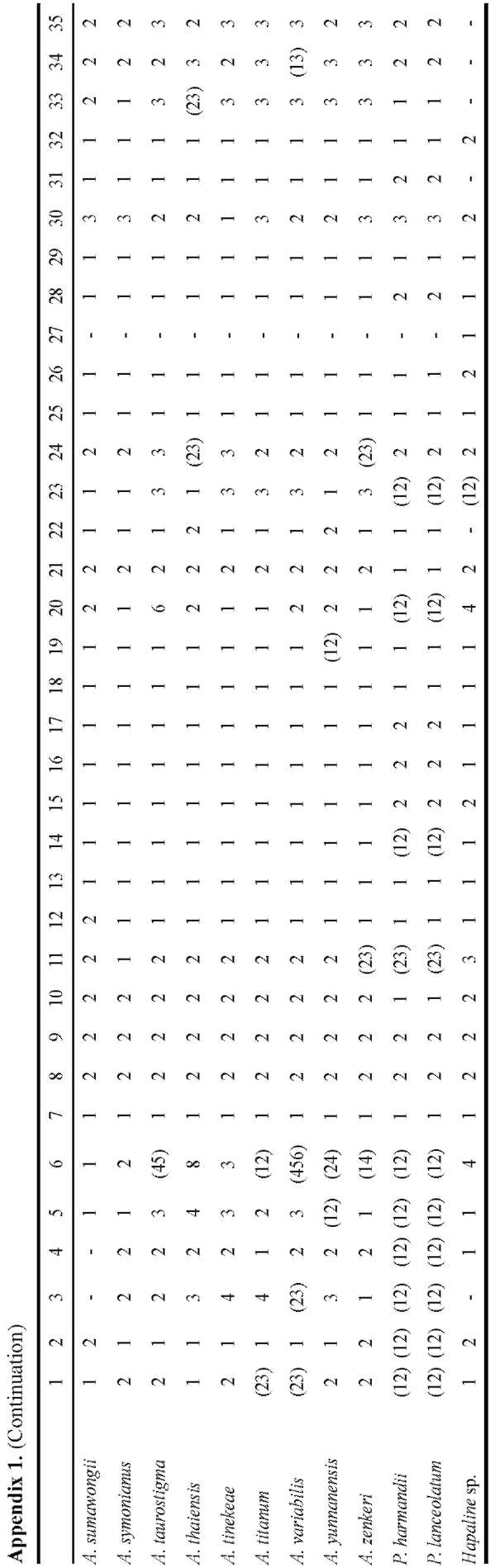
A matrix of 70 morphological characters was used to reconstruct character evolution (Table 2). Morphology characters were plotted on the MP tree with the highest likelihood score (Figure 3) using the assumptions of MP with the trace character command (ACCTRAN optimisa-
|
|
|
|
|
|
|
|
The rbcL gene was amplified and sequenced with
primers 1F (ATGTCACAACAAACAGAAAC),
724R (GCGTTGGAGAGATCGTTTCT), 636F
(TCGCATGTACCTGCAGTAGC) from Fay et al.
(1997) and 1460R from Olmstead et al. (1992). The
trnL intron was amplified using universal primers
"c" (CGAAATCGGTAGACGCTACG) and "d"
(GGGGATAGAGGGACTTGAAC) (Taberlet et al.,
1991). The second intron of LEAFY was amplified with the primers FLint2 F1 (CTTCCACCTCTACGACCAGTG) and FLint2 R1 (TCTTGGGCTTGTTGATGTAGC) (Grob
et al., 2004).
Amplifications were done with a Biometra Thermocyler T3. A 50 μL reaction mix was composed of 41.4 μL milliQ water, 5 μL PCR buffer, 2 μL dNTP,s 0.2 μL (25 μM) primer forward and reverse, 0.2 μL Taq polymerase and 1 μL (10x diluted containing ca. 5 ng DNA) template for rbcL. The trnL mix consisted of 6 μL miliQ water, 5 μL PCR buffer, 2 μL dNTP,s, 0.5 μL (25 μM) primer forward and reverse, 0.4 μL Taq polymerase and 1 μL (10x diluted) primer for a total of 50 μL mix. The LEAFY mix consisted of 36 μL milliQ water, 5 μL buffer, 2 μL dNTP,s, 0.2μL (25 μM) primer forward and reverse, 0.2 μL Taq polymerase, 1 μL (undiluted) primer and 5 μL MgCl2. The
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus
|
|
|
|
|
|
|
|
|
|
tion) m MacClade version 4.06 (Maddison and Maddison, 2003).
|
|
|
|
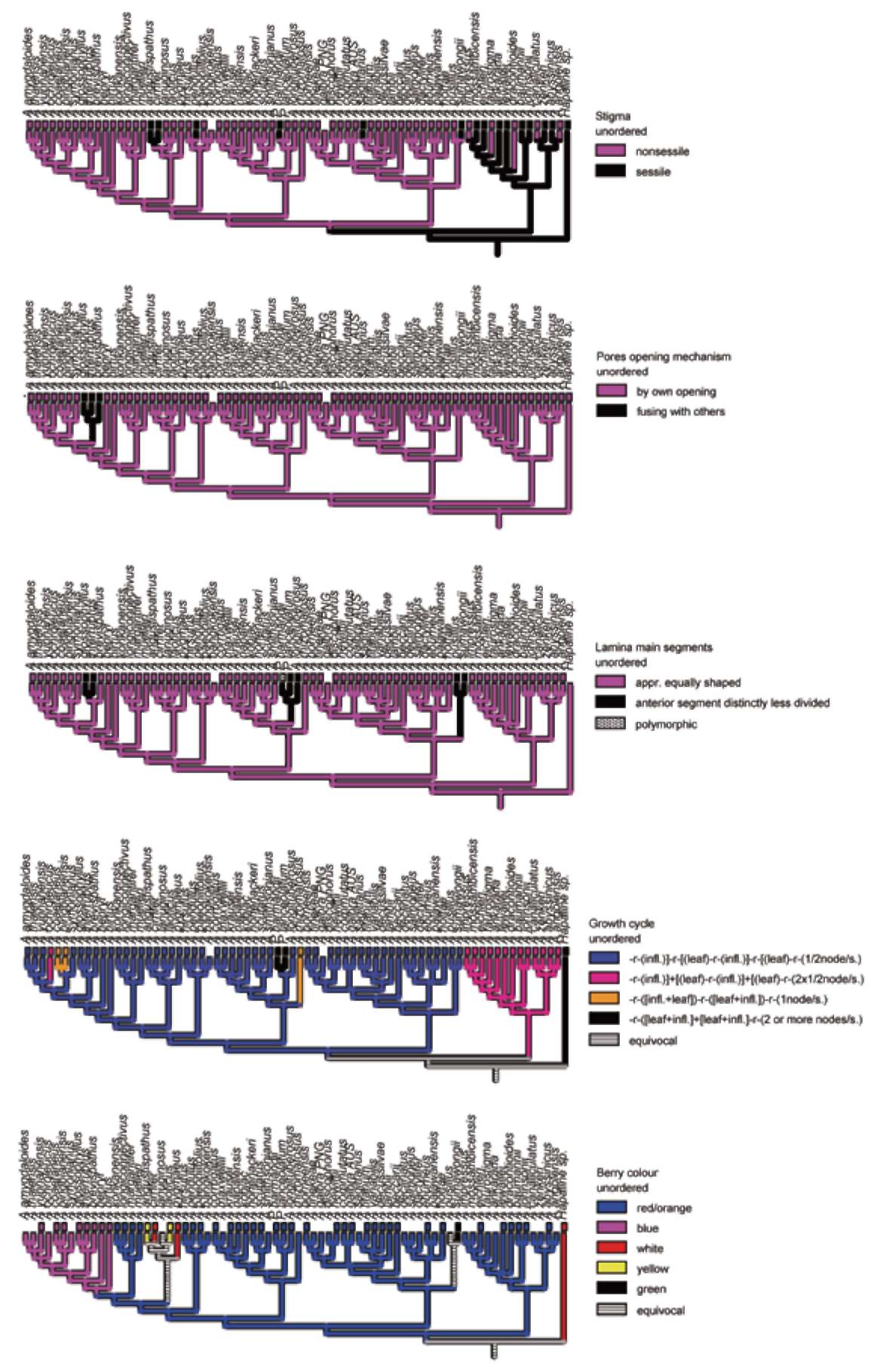
Optimised morphological characters (Figure 4)
|
|
|
|
Five characters were used for optimisation. The first one was stigma morphology (Table 2, char. 2): styles in Amorphophallus vary from almost absent (stigma directly sessile on the ovary) to very long and thin (stigma nonsessile). The second was the opening mechanism of the pores (Table 2, char. 18): in most Amorphophallus species pores open independently from each other per anther. In a few species the connectival tissue is very thin and ruptures when the pores open. Adjacent pores then "fuse" into a larger pore, discharging pollen from two adjacent thecae. The third character was the lamina architecture (Table 2, char. 49): decompound lamina in Amorphophallus usually have three easily recognisable main segments. These are usually equal in shape and complexity, but in several species the single posterior segment is much less complexely divided and often shorter than the laterals. The fourth character was the growth cycle (Table 2, char. 58): most Amorphophallus species show sympodial cyclic growth with a resting period alternating with an active growing period after a shorter or longer seedling period of monopodial growth, terminated by the first occasion of flowering. During the resting period (identified as -r-in the states of character 58) no plant parts are above soil. Two out of four different sympodial cycle types are dominant. In one or these, a single inflorescence develops on a node, and afterwards the plant goes dormant again without leaf development for the rest of the season (char. 58, state 1). A single leaf emerges in the next season, but no inflorescence. The leaf devours the old node and builds a new one. This is the most common cycle type and is found only in Asian species. The other common type shows development of an inflorescence preceding that of a leaf in the same season, with the leaf again devouring the old node and building a new one. This cycle-type is found exclusively in all African species. The two other cycle types also show flowering and leaf development in the same season but with different timing of node-development relative to the African cycle-type (Table 2, char. 58, states 3 and 4). The last character optimised was berry colour (Table 2, char. 65): of all Araceae genera, Amorphophallus shows the highest diversity in colour of the mature berries with red/orange being dominant. Other colours are white, blue, purple, yellow, green, and brown.
|
|
|
|
|
|
|
|
|
|
|
|
The rbcL sequence alignment consisted of 1512 characters, of which 1347 characters were constant, 79 were parsimony-uninformative, 86 were informative, and 7 were indels. The trnL alignment consisted of 963 characters and included 28 indels. A region of TA repeats with a total length of 207 bp in A. hohenackeri
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
Table 2. Morphological characters and character states used in morphological character optimization.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1. Ovary (number of locules): 1 one; 2 two; 3 three; 4 four.
2. Stigma: 1 nonsessile; 2 sessile.
3. Stylar length (relative to ovary): 1 much shorter (almost nil); 2 shorter (appr. 0.5x); 3 士 equal; 4 distinctly longer (2x and
more).
4. Stigma diameter (relative to style-diam.): 1 士 equal; 2 larger.
5. Stigma: overall shape in longitudinal section: 1 depressed; 2 globose / hemispheric; 3 conical; 4 obconical.
6. Stigma: structure: 1 entire; 2 with central depression; 3 one-lobed; 4 two-lobed; 5 three-lobed; 6 four-lobed; 7 multi-lobed; 8 bilabiate (folded); 9 士 cup shaped.
7. Placentation: 1 basal; 2 axillary, halfway up the length of the placenta.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8. Average number of stamens per flower: 1 one; 2 (2-)3-5(-6); 3 more (indeterminate).
9. Filaments: 1 (near-)absent (anthers 士 sessile); 2 present.
10. Filaments: shape: 1 slender; 2 thick.
11. Filaments (majority): fusion: 1 entirely free; 2 partly connate, sometimes forming a column; 3 entirely connate, forming a column or cushion.
12. Lower filaments: transforming to staminodes (1): 1 not transformed; 2 filaments swollen & fused; 3 filaments swollen & fused, anthers absent (flowers fully staminodial).
13. Lower male flowers: reduction transformation (to hairs): 1 not transformed; 2 transformed.
14. Anthers (majority): fusion: 1 free; 2 fused.
15. Anther-shape: 1 rectangular/cubic; 2 globose/subglobose/hemispheric.
16. Thecae: 1 bilocular; 2 unilocular.
17. Pores: position: 1 apical; 2 lateral; 3 subapical.
18. Pores; opening mechanism: 1 by own opening; 2 fusing with others, connective rupturing.
19. Connective: shape: 1 flat; 2 ridge-like; 3 elongate (vertically); 4 hemispheric.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20. Pollen: exine ornamentation: 1 psilate; 2 striate; 3 verrucate; 4 echinate; 5 areolate; 6 fossulate; 7 reticulate; 8 striate/scabrate; 9 scabrate.
21. Polar caps: 1 present; 2 absent.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22. Spadix: 1 (sub-)sessile; 2 distinctly stipitate.
23. Length relative to spathe: 1 distinctly shorter than spathe; 2 ±equalling spathe; 3 longer than spathe.
24. Female zone: length rel. to male zone: 1 much shorter than male zone (less than 0.2x); 2 shorter than male zone (0.2 - 0.9x); 3 ±equalling male zone; 4 longer than male zone (1.1 - 1.9x); 5 much longer than male zone (2.0x and more).
25. Female zone: fertility: 1 entirely fertile; 2 with staminodia.
26. Female to male zone: 1 adjacent (contiguous); 2 separated by sterile zone.
27. Sterile zone between male and female zone: 1 (largely) naked (sometimes with flower remains); 2 with sterile structures (staminodes or pistillodes).
28. Male zone: disposition stamens: 1 congested or slightly distant; 2 loosely arranged; 3 aligned/fused into vertical ridges; 4 aligned/fused into a lax spiral; 5 (sub)verticillate/dense spiral.
29. Upper part of male zone: fertility: 1 entirely fertile; 2 with interspersed sterile structures (hairs).
30. Male zone: diam. relative to female zone: 1 distinctly narrower than fem. Zone; 2 appr. equalling female zone; 3 distinctly exceeding fem. Zone.
31. Male zone: transition to appendix: 1 contiguous; 2 naked zone, distinct from appendix ("stipe").
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32. Presence: 1 present; 2 absent.
33. Appendix: length rel. to male ±fem. zone: 1 shorter; 2 士 equal; 3 longer.
34. Appendix: diameter (base) rel. to male zone: 1 less than in male zone; 2 ±equal or slightly broader; 3 exceeding.
35. Appendix: general shape (lateral view): 1 ovoid/globose (1:1 - ca. 1.5:1); 2 shortly conical (ca. 2:1); 3 elongate (2.5:1 or more).
36. Wall structure: 1 individual elements (staminodes) visible; 2 no elements visible, or only at the base or top.
37. Wall: elements (staminodes): 1 verrucae; 2 high ridges/columns (incl. papillae/short ridges); 3 shallow ridges/colums; 4 broad, flat colums; 5 hairs; 6 bristles._
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
38. Wall, high ridges: shape: 1 long (rugae); 2 short (papillae).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
39. Transition base-limb: 1 gradual, not or shallowly constricted; 2 distinctly constricted.
40. Spathe base: surface within: 1 smooth; 2 sculptured.
41. Spathe base: sculpture type: 1 small (simple) verrucae; 2 large verrucae (sometimes irregular); 3 papillae (sometimes hairlike); 4 hairs; 5 ridges; 6 verrucae-ridges (intermediates); 7 transverse ridges.
42. Spathe base: degree of sculpturing: 1 dense/numerous; 2 scattered/few.
43. Spathe base: 1 short and loosely convolute; 2 strongly convolute, forming a basal tube/chamber; 3 margins fused.
44. Limb: apex: 1 acuminate; 2 acute; 3 obtuse.
45. Limb: margin shape: 1 straight; 2 undulate; 3 plicate/strongly folded.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
46. Length rel. to spathe: 1 shorter than or equalling spathe; 2 longer than spathe.
47. During fruiting: 1 elongating; 2 no substantial growth.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
48. Duration: 1 short lived (1 season, between two dormancy periods); 2 long lived (2 - 5 years).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
49. Main segments: 1 appr. equally shaped; 2 anterior segment distinctly less divided.
50. Main posterior segment: division: 1 moderately subdivided; 2 strongly subdivided.
51. Leaflets (all): base: 1 all/most sessile; 2 basal ones distinctly petiolulate.
52. Leaflets upper surface colour: 1 green/grey-green; 2 deep velvety green.
53. Leaflets variegation: 1 all green (no variegation); 2 heavily splashed with white dots; 3 white line along midrib (and occ. sec. veins); 4 greyish area along midrib; 5 scattered white spots; 6 checkered.
54. Leaflets margin: 1 green; 2 violet/lilac.
55. Rhachises: 1 winged throughout; 2 winged distal from the main basal branchings; 3 (largely) unwinged.
56. Foliar bulbils: 1 absent; 2 present.
57. Foliar bulbils: dislodging: 1 dislodging completely from rachis; 2 dislodging with parts of the rachis.
58. Growth cycle [] = node; () = season; r = rest: 1 -r-(infl.)]-r-[(leaf)-r-(infl.)]-r-[(leaf)-r- (1/2 node/s.); 2 -r-(infl.]+[leaf)-r-(infl.]+[leaf)-r- (2 x 1/2 node/s.); 3 -r-([leaf+infl.])-r-([leaf+infl.])-r- (1 node/s.); 4 -r-([leaf+infl.]+[leaf+infl.)-r- (2 or more nodes/s.)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
59. Number of modules: 1 one; 2 more than one.
60. Orientation: 1 vertical; 2 horizontal.
61. Module shape: 1 saucer shaped (extremeley depressed); 2 globose/depressed-globose; 3 elongate (very narrow, growing apex with numerous cataphylls or -scars); 4 elongate (thick, no cataphylls or -scars).
62. Underground vegetative propagation: 1 only by division (break-up) of mature tuber/rhizome; 2 by development of subsidiary tubers (slowly separating); 3 by offset-development (usually seasonal).
63. Offsets: shape: 1 globose; 2 shortly elliptic/fusiform; 3 shortly cylindric; 4 distinctly elongate (rhizomatous); 5 obconic (= globose with short "stalk").
64. Root-scars: 1 with distinct, annular thickenings (abscission); 2 without annular thickenings.
65. Berries: colour: 1 red/orange; 2 blue; 3 white; 4 yellow (with our without white base); 5 green; 6 pale brownish (soil colour); 7 purple.
66. Berries: surface: 1 smooth; 2 ridged/verrucate.
67. Spathe at anthesis: 1 opening, separating from spadix; 2 clasping the spadix.
68. Spathe: overall colour intensity/-type inside: 1 pale (whitish, greenish, pinkish); 2 dark (purple, blackis-grey).
69. Spathe margin: 1 straight; 2 broadly rounded/curved inwards; 3 sharply curved inwards-channeled.
70. Number of inflorescences per flowering event: 1 one inflorescence; 2 more than one (in synflorescence).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
was excluded from the analysis. Among the 756 included characters, 634 were constant, 74 were parsimony-uninformative, and 48 are parsimony-informative. The LEAFY alignment consisted of 591 characters and included 67 indels. Two regions of 70 bp in A. angustispathus and A. konkanensis and 29 bp in A. hohenackeri, respectively, were considered unalignable, and therefore excluded from the analysis. Of the 492 included characters, 257 were constant, 88 were parsimony-uninformative, and 147 were parsimony-informative. The combined matrix consisted of 3066 characters. A total of 306 characters were excluded, leaving 2760 characters in the analysis, of which 2238 were constant, 241 were parsimony-uninformative, and 281 are parsimony-informative. Among the three genes, LEAFY generated the most variation, followed by trnL. The rbcL gene was found to be a much more conserved region.
|
Maximum parsimony and maximum likelihood analyses
|
|
|
|
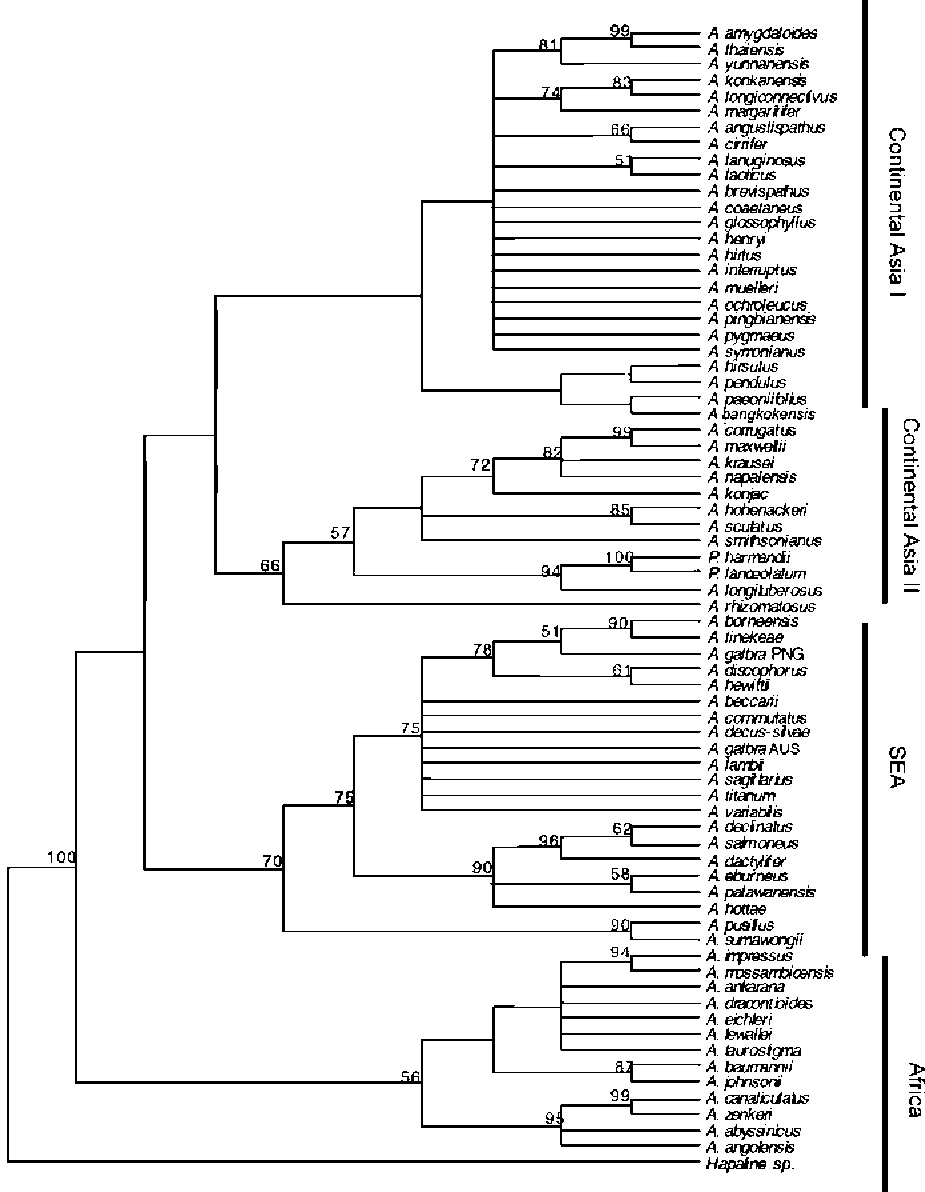
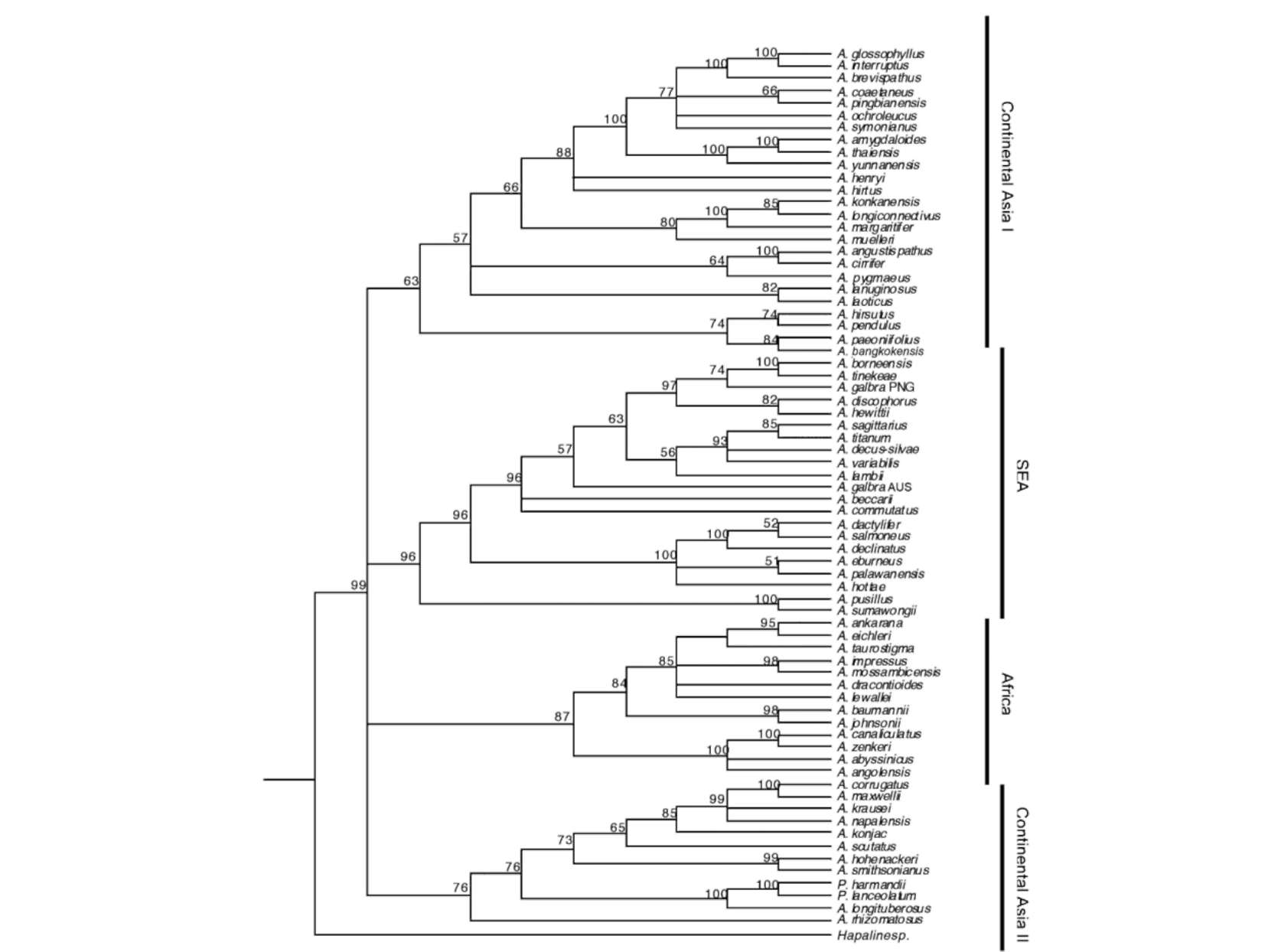
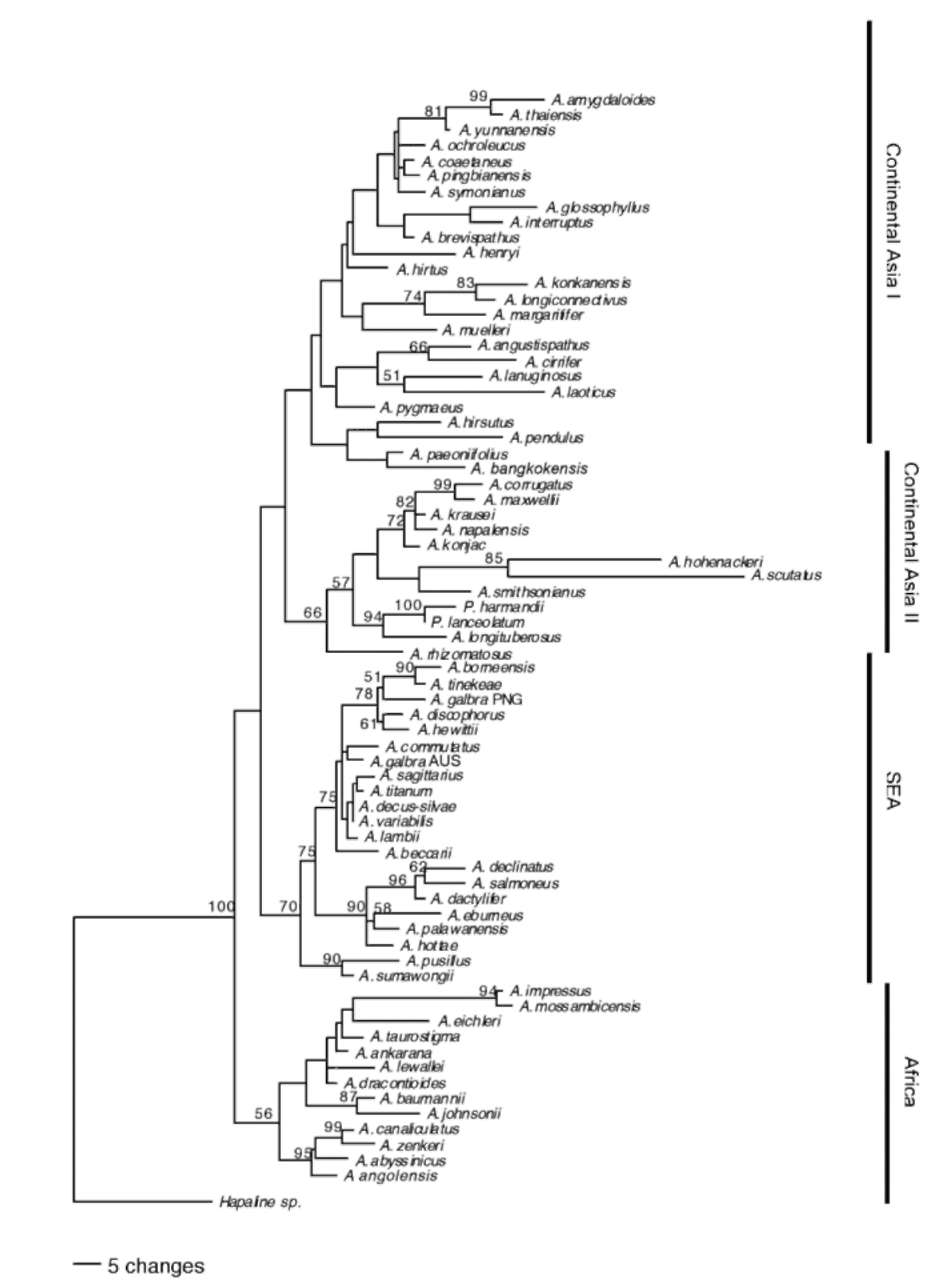
All three MaxTree settings produced the same parsimony scores with a tree length of 1030 steps, CI=0.590, RI=0.701, and RC=0.414. Compared to the MP strict consensus presented by Grob et al. 2002, 2004, the MP tree presented in this study has a better resolution both for basal and terminal clades (Figure 1). In total, 22 ingroup nodes are supported by >70% BS. The MP strict consensus contains three major clades, namely African, Southeast Asia (SEA) and Continental Asia clades. The African clade comprises 13 species and is supported by 56% BS. The SEA clade comprises 21 species from the Indo-Pacific archipelago, the Philippines, Papua New Guinea, and Australia and is supported by 70% BS. The biggest major clade (<50% BS) in the MP strict consensus is the Continental Asia clade, which covers the taxa distributed from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 1. Maximum Parsimony strict consensus of 253,317 MPTs. BS values are given above the nodes.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus 481
|
|
|
|
|
|
India to China, Taiwan, and Thailand. This clade contains two species, A. hirsutus (Sumatra) and A. pendulus (Borneo) which do not occur on continental Asia. The clade is subdivided into two subclades, Continental Asia I (<50% BS) and II (66% BS), which reflect no biogeographical distinction. They are, however, supported by unique berry colours.
|
polytomy. In the tips, however, the Bayesian consensus tree was better resolved than the strict consensus tree.
|
|
|
|
|
|
Each method used has its own advantages and disadvantages. Maximum Parsimony is capable of analyzing large sequence datasets, but can perform poorly if there is substantial variation in branch lengths. Bayesian analysis is better suited for tackling such branch length variation, but the prior distributions for parameters must be specified, and it is difficult to decide whether MCMC has run long enough before reliable results are obtained (Holder and Lewis, 2003). All resulting cladograms from both analyses are congruent in several parts, though, and apart from their geographical coherence, the larger clades can be defined by some morphological synapomorphies. First of all, the
|
|
|
|
|
|
With MrModeltest 1.1b (Nylander, 2002), two models
of nucleotide substitution, GTR+G+I for rbcL and HKY+G for both trnL and LEAFY were selected. The Bayesian analysis resulted in a 50% majority consensus tree (Figure 2) with one major topological difference as compared to the MP strict consensus obtained. Along the backbone of the tree, three major clades collapsed into a
|
|
|
|
|
|
|
|
|
|
|
|
Figure 2. Bayesian 50% majority rule consensus tree. Posterior probability values are given above the nodes.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
monophyly of African clade is supported by all analyses (Figures 1, 2, 3). The African species are characterized by a unique seasonal growth cycle in which two tuber nodes are active within a single season. These nodes have a differential development: the first produces an inflorescence and the second a leaf (Table 2, char. 58, state 2).
Secondly, in the MP and Bayesian analyses (Figures 1, 2), A. galbra from PNG is placed in a different clade as compared to A. galbra from Australia. The sequences are derived from plants with conspicuously different vegetative morphologies. The PNG specimen is a large plant up to two meters high. The petiole and peduncle bear crusty patches resembling lichens and are generally multicolour-ous. The spathe is multicolourous. The Australian speci-
|
men, on the other hand, is a small plant, not higher than 30 cm. The petiole and peduncle are entirely green and smooth. The spathe is entirely green. As both specimens have a very different morphology and end up in very different phylogenetic positions both in the MP and Bayesian trees, it suggests that A. galbra needs further taxonomic revision and perhaps a redefinition of its species boundaries.
Thirdly, A. paeoniifolius and A. bangkokensis always cluster together, despite their considerable vegetative morphological differences. A. paeoniifolius is a plant of which the tuber has thick annular root scars, the offsets are very short and thick, the petiole is usually strongly warty, rarely smooth, and the petiole is always blotched
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 3. The Maximum Parsimony tree of 253,317 trees with the highest likelihood score (-ln L = 9144.6). Length=1030 steps, CI=0.590, RI= 0.701, RC=0.414. BS values are given above the nodes.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus
|
|
|
|
|
|
|
|
|
and never striped. In contrast, A. bangkokensis has a tuber lacking annular root scars, its rhizomatous offsets are long and thin, and the petiole is smooth with a stripe-like pattern. The inflorescences of both species are very much alike although that of A. bangkokensis is consistently small (spadix not longer than 15 cm) and that of A. paeoniifolius is hardly ever that short but may reach up to 50 cm.
Fourthly, the clade of A. pusillus (Vietnam) and A. sumawongii (Thailand), which was also found by Grob et al. (2002, 2004) was recovered in all analyses of this study as well. It is supported by several morphological characters.
|
of the connective, which has been transformed in these species into a very thin tissue layer partly covering the pores. Simultanously the two pores on a theca also merge into one because of the rupturing of the connective. It would be interesting to investigate whether these species also have a unique pollen dispersal method as compared with the remaining species sampled. Pollen release by connective rupturing seems to be derived from pollen released directly from the pores.
|
|
|
|
|
|
|
|
The leaf lamina in Amorphphallus is called "decompound" because it is split up in three main segements, each usually branching to various degree and carrying highest order segments shaped as individual small leaves ("leaflets"). In most species sampled, the main segments of the leaf lamina are more or less equally shaped. Distinctly less divided anterior main leaf segments evolved from equally shaped segments three times in the Asian clades. From an ontogenetic point of view, it has not been possible yet to determine if these segments have an arrested development. Evo-devo studies could shed further light on the homology of these structures. It is suggested that this phenomenon in the Pseudodracontium species may be the result of hetero-chronic development because other plant parts show similar phenomena (e.g. staminodial appendix, male flowers with long thin filaments and separated anthers, unilocular thecae; Hetterscheid and Claudel, in prep.)
|
|
|
|
|
|
|
|
Resolving the basal backbone of the MP strict consensus has shed more light on some aspects of evolution of the genus Amorphophallus. The African and Southeast Asian clades seem well defined, despite the presence of the Indian species A. commutatus in the latter which seems better fitted in the continental Asian clade. The continental Asian clade is more difficult to interpret since it covers both a wide geographical range and large morphological variation.
|
|
|
|
Morphological character evolution
|
|
|
|
With MacClade, character evolution was optimized most parsimoniously using ACCTRAN optimization.
When plotted on the MP tree with the highest likelihood score, some morphological characters were found to correlate well with molecular based clades. These characters include a stigma (either nonsessile or sessile), a pore opening mechanism, the shape of the main segments of the leaf lamina, the growth cycle, and a berry colour (Figure 4).
|
|
|
|
|
|
|
|
The African clade is well supported by a unique growth cycle where the season's growth starts with a developing inflorescence and the leaf appearing either after a short while or almost simulaneously. The appearing inflorescence terminates the sympodial shoot initiated the season before while the leaf developing alongside it in the same season develops from a lateral continuation shoot and initiates the next sympodial cycle. The dormancy period of an individual plant therefore occurs in the middle of the development of a sympodial module from leaf initiation to inflorescence termination. One season of growth is characterized by the termination of the previous year's module and the initiation of the next, which is not terminated that season by another inflorescence but undergoes a dormancy period and is later terminated by an inflorescence in the next season. Therefore inflorescence and leaf appearing in the same growing season do not belong to one and the same sympodial module but to two consecutive ones separated by a dormancy period.
A seemingly similar situation of simultaneously existing leaf and inflorescence in the same season is also found in a few continental Asian species like A. ochroleucus, A. coaetaneus and A. rhizomatosus. However, in these instances the leaf appears first, and several weeks or months later the inflorescence appears, and the latter is the termination of the same shoot from which the leaf has developed. This situation repeats itself the next season.
|
|
|
|
|
|
|
|
The African clade and outgroup seem to be characterized by a sessile stigma, with the exception of A. impres-sus, A. taurostigma, A. dracontiodes, A. canaliculatus and A. angolensis which have a nonsessile stigma. A nonsessile stigma can be found in all other species except for A. cir-rifer, A. angustispathus, A. pendulus, and A. sagittarius. A nonsessile stigma seems to be evolved from a sessile one. The morphology of the stigma (either sessile or nonsessile) is phylogenetically informative for the entire Araceae family (Mayo et al., 1997), and it would be interesting to investigate whether nonsessile stigma's also developed in selected clades only during the evolution of Amorphophal-lus when a fully sampled and resolved phylogeny of the genus becomes available.
|
|
|
|
|
|
|
|
All species sampled seem to be characterized by pollen being released through individual pores, with the exception of A. glossophyllus, A. interruptus and A. brevispathus which have pollen realeased after a rupturing
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 4. Reconstruction of character state evolution of several morphological characters optimized on the MP tree with the highest likelihood, using the trace character command in MacClade version 4.06 (Maddison and Maddison, 2003).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus 485
|
|
|
|
|
|
Therefore, leaf and inflorescence of one season's growth belong to the same sympodial module. Both these cycle types differ from the most common one (present in most Asian species), in which each season's growth sees either an inflorescence or a leaf developing, but never in the same season.
This latter type of growth cycle is reconstructed to be ancestral to the one in e.g. A. ochroleucus, whereas it cannot be decided if the dominant Asian cycle type is ancestral to the African species cycle or the reverse. The optimisation shows ambiguity at this point. A large variation in growth cycles exist within the Araceae family
(Scribailo and Tomlinson, 1992; Mayo et al., 1997).
It would be very interesting to study these different types of inflorescence, leaf, and shoot development in a phylogenetic context for the whole family.
|
lus presented here, more insight could be obtained about the evolution of ecologically interesting features such as dwarfism, life cycle, and berry colour. A next step would be to unravel the genetics of these features by crossing species from sister groups with different habit sizes, life cycles, and berry colours for quantitative trait loci (QTL) analysis. Although nothing is known about the genetics of these features yet, it seems feasible that only a small number of genes is involved in their initiation and development because of the recurrent evolution of the same type of life cycle and berry colour in Amorphophallus.
|
|
|
Acknowledgements. We thank Mr. Art Vogel and Mrs. Hanneke Jellema for taking care of the cultivated plants at the Hortus Botanicus Leiden and Delia Co, Hugh Cross, Peter Hovenkamp and Lynn McIvor for help with the phylogenetic analyses. This research was funded by a STUNED scholarship, provided by The Netherlands Education Centre, Jakarta, Indonesia as part of the second author's requirement of an MSc degree at the Nationaal Herbarium Nederland - Leiden University.
|
|
|
|
|
|
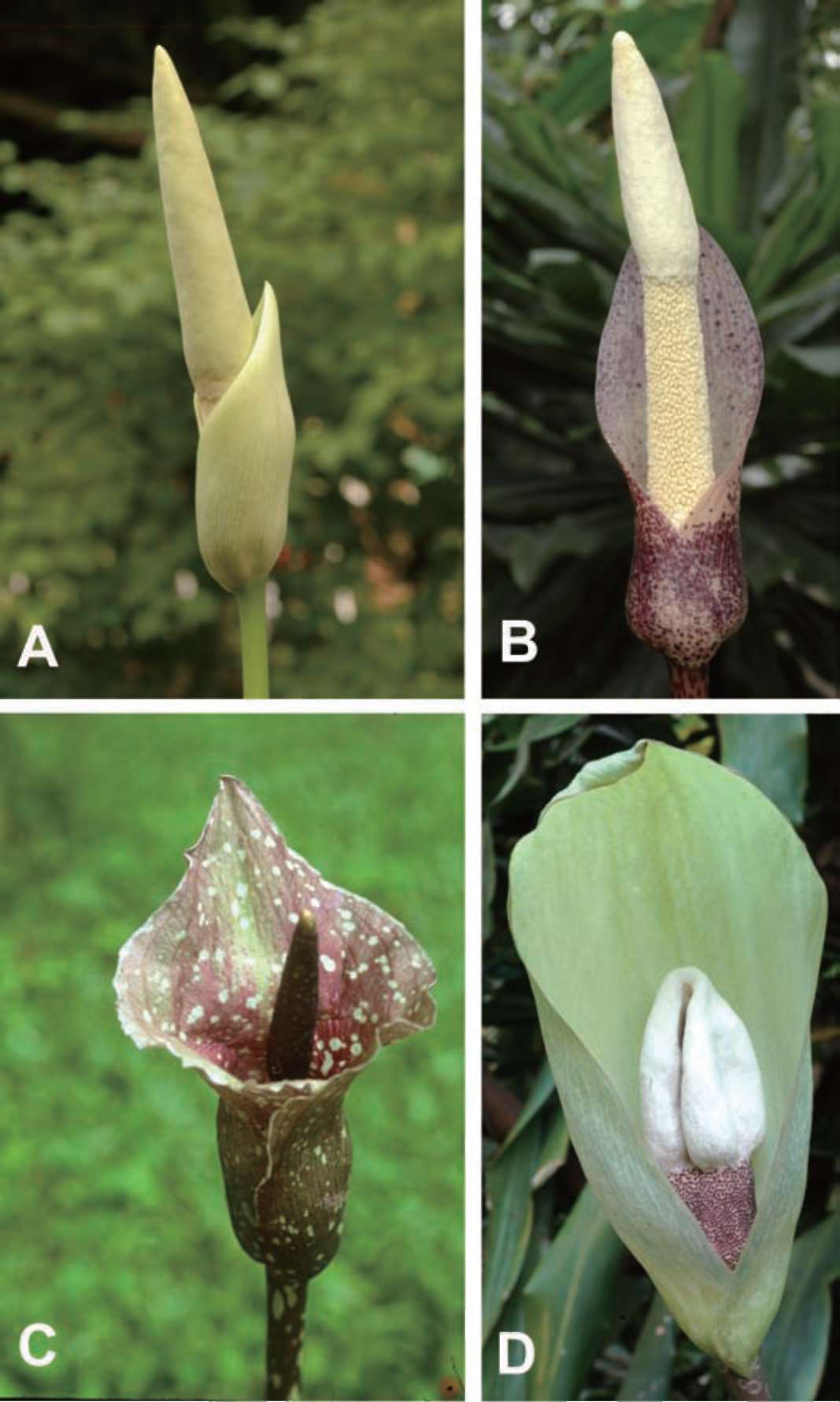
A clade of 13 taxa in the Continental Asia I clade shows the possession of blue/purple berries, which do not occur in other clades. Blue berries occur in the taxa with the northernmost distribution of Amorphophallus and might have evolved as a response to birds that focus on blue berries for food in that particular geographical region (Hetterscheid and Ittenbach, 1996). Blue/purple, green, white, and yellow berries are all reconstructed as derived from red/orange or white berries. It is interesting to note how variable the inflorescence morphology is for species within the blue-berried clade, with species like A. brevispathus, A. coaetaneus, A. kiusianus and A. yun-nanensis (see Plate 1). From a macromorphological point, this clade would not be retrievable, and this is proven in such an analysis (Hetterscheid and Hovenkamp, in prep.). Observations like this indicate the great morphological flexibility in Amorphophallus, which may well be due to a strong adaptibility to different pollination resources (Hetterscheid, in prep.). The clade containing the smallest species of Amorphophallus~jA. obscurus (not analysed here), A. polyanthus (not analysed here), A. pusillus, A. serrulatus (not analysed here) and A. sumawongii (A. pusillus having an inflorescence of a mere 5 cm high―is further characterised by all species sharing an atypical leaflet-structure (rhombic - obovate) and verrucate berries with a very unorthodox colour (green in A. sumawongii; dirty pinkish-brownish in A. polyanthus). Ecologically, the members of this group seem to be adapted to a survival strategy suitable to forest floor conditions indicated by the type of pollination (fungus syndrome; Kite and Hettersc-heid, 1997) and type of dispersal (berries without striking, bird-attracting colours and infructescence held close to the soil). The position of this small clade of purely Thai-In-dochinese species in the large Southeast Asia clade is not supported by morphology, especially because of the presence of elongate tubers, a character very common in other Thai-Indochinese species but absent in all other Southeast Asian species.
With the three-gene-based phylogeny of Amorphophal-
|
|
|
|
|
|
Blume, C.L. 1835. Tribe Thomsoniae. Rumphia 1: 138-150.
Bogner, J., S. Mayo, and M. Sivadasan. 1985. New species and changing concepts in Amorphophallus. Aroideana 8: 14-25.
Cabrera, L.I., G.A. Salazar, M.W. Chase, S.J. Mayo, J. Bogner, and P. Davila. 2008. Phylogenetic relationships of aroids and duckweeds (Araceae) inferred from coding and noncod-ing plastid DNA. Amer. J. Bot. 95(9): 1153-1163.
Cho, Y. and J. D. Palmer. 1999. Multiple acquisitions via horizontal transfer of a Group 1 intron in the mitochondrial coxl gene during evolution of the Araceae family. Mol. Biol. Evol. 16(9): 1155-1156.
Fay, M.F., S.M. Swensen, and M.W Chase. 1997. Taxonomic
affinities of Medusagyne oppositifolia (Medusagynaceae).
Kew Bull. 52: 111-120.
Frohlich, M.W. and E. M. Meyerowitz. 1997. The search of homeotic gene homologs in basal angiosperms and Gnetales: a potential new source of data on the evolutionary origin of flowers. Int. J. Plat. Sci. 158: S131-S142.
Grob, G.B.J., B. Gravendeel, M.C.M. Eurlings, and W.L.A.
Hetterscheid. 2002. Phylogeny of the tribe Thomsoniae (Araceae) base don Chloroplast matK and trnL intron sequences. Syst. Bot. 27: 453-467.
Grob, G.B.J., B. Gravendeel, and M.C.M. Eurlings. 2004.
Potential phylogenetic utility of the nuclear FLORICAULA/ LEAFY second intron: comparison with three chloroplast DNA regions in Amorphophallus (Araceae). Mol. Phyl.
Evol. 30: 13-23.
Hetterscheid, W.L.A. and S. Ittenbach. 1996. Everything you always wanted to know about Amorphophallus but were afraid to stick your nose into. Aroideana 19: 7-129.
Holder, M. and P.O. Lewis. 2003. Phylogeny estimation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
traditional and Bayesian approaches. Nature Rev. Genetics 4: 275-284.
Jannsen, T. and K. Bremer. 2004. The age of major monocot groups inferred from 800+ rbcL sequences. Bot. J. Linn.
Soc. 146: 385-398.
Kite, G.C. and W.L.A. Hetterscheid. 1997. Inflorescense odours of Amorphophallus and Pseudodracontium (Araceae). Phytochemistry 46: 71-75.
Maddison, W.P. and D.R. Maddison. 2003. MacClade version 4.06. Sinauer Associates, Sunderland.
Mayo, S.J., J. Bogner, and P.C. Boyce. 1997. The genera of Araceae. Royal Botanic Gardens, Kew.
Nylander, J.A.A. 2002. Testing models of evolution― MrModeltest version 1 . 1 b. Computer program and documentation distributed by author, website: http:〃www. ebc.uu.se/systzoo/staff/nylander.html.
Oh, S.-H. and D. Potter. 2003. Phylogenetic utility of the second intron of LEAFY in Neillia and Stephanandra (Rosaceae) and implications for the origin of Stephanandra. Mol. Phyl.
Evol. 29: 203-215. Olmstead, R.G., H.J. Michaels, K.M. Schott and J.D. Palmer.
1992. Monophyly of the asteridae and identification of their major lineages inferred from DNA sequenced of rbcL. Ann. Missouri Bot. Gard. 79: 249-265.
|
Ronquist, F. and J.P. Huelsenbeck. 2003. MrBayes 3. Bayesian phylogenetic inference under mixed models. Bioinformatics
19: 1572-1574.
Rothwell, G.W., M.R. Van Atta, H.E. Ballard Jr., and R.A. Stockey. 2004. Molecular phylogenetic relationshipsamong Lemnaceae and Araceae using the chloroplast trnL-trnF intergenic spacer. Mol. Phyl. Evol. 30: 378-385.
Scribailo, R.W. and P.B. Tomlinson. 1992. Shoot and Floral Development in Calla palustris (Araceae-Calloideae). Int. J.
Plant Sci. 153: 1-13 Soltis, P.S. and D.E. Soltis. 2003. Applying the bootstrap in
phylogeny reconstruction. Stat. Sci. 18: 256-267.
Taberlet, P., L. Gielly, G. Pautou, and J. Bouvet. 1991. Universal primers for amplification of three non-coding regions of
chloroplast DNA. Plant Mol. Biol. 17: 1105-1109.
Tamura, M. N., J. Yamashita, S. Fuse, and M. Haraguchi. 2004. Molecular phylogeny of monocotyledons inferred from combined analysis of plastid matK and rbcL gene sequences. J. Plant. Res. 117: 109-120.
Wikstrom, N., V. Savolainen, and M.W. Chase. 2001. Evolution of the angiosperms: calibrating the family tree. Proc.R. Soc.
Lond. B. 268: 2211-2220.
|
|
|
|
|
|
|
|
依據trnL, rbcL與LEAFY第二內插子序列之親緣分析
推論天南星科魔芋屬植物之形態演化
|
|
|
|
|
|
|
|
Agung SEDAYU2, Marcel C. M. EURLINGS3, Barbara GRAVENDEEL3 and Wilbert L. A. HETTERSCHEID1
|
|
|
|
|
|
|
|
1 National Herbarium of The Netherlands, Wageningen University branch, Wageningen University Botanical Gardens (presently: Von Gimborn Arboretum, Doorn, Netherlands)
2 Bogor Botanical Gardens, Indonesia
3 Netherlands Centre for Biodiversity Naturalis, National Herbarium of The Netherlands, Leiden University
|
|
|
|
|
|
|
|
本研究使用69個分類群,結合三個不同的基因序列以重建富含眾多物種的天南星科魔芋屬植物的
分子親緣關係。序列資料集以最儉約法、最大似然法與貝葉氏分析三種不同的方法產生了三個稍微不同
的親緣樹。所有的分析顯示了反映魔芋屬的地理分布關係的三個主要枝系。有的枝系得到形態特徵上的
支持,例如柱頭是否具柄、花粉釋出機制、葉身主裂片的形狀、生長周期與漿果顏色。在特徵演化的最
優化之下顯示具柄的柱頭可能是演化自無柄柱頭物種,並經歷數次逆轉演化;花粉釋出機制為藥隔破裂
演化自孔裂花藥;葉身形狀不對稱的裂片演化自對稱的裂片;葉與花序同時存在者是從葉與花序不同時
出現者演化而來;具有藍色、紫色、綠色與黃色的漿果是由紅色、橙色或白色的漿果演化而來。
|
|
|
|
|
|
|
|
關鍵詞:魔芋屬;天南星科;特徵最佳化;LEAFY基因;分子親緣;trnL序列;rbcL基因。
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plate 1. Amorphophallus species of the "blue berry"-clade: A, A. brevispathus; B, A. ochroleucus; C, A. kiusianus; D, A. yunnanensis. (A, B, D: photos by last author; C: photo by C.-I Peng).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEDAYU et al. ― Morphological character evolution in Amorphophallus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 51, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|