Botanical Studies (2011) 52: 15-22.

Sweet potato storage root thioredoxin h2 and their peptic hydrolysates exhibited angiotensin converting enzyme inhibitory activity in vitro
Guan-Jhong HUANG1,8, Hsien-Jung CHEN2,8, Kitanaka SUSUMU3, Jin-Bin WU4, Wen-Chi HOU5, Chieh-Hsi WU6, Ming-Jyh SHEU6, Shyh-Shyun HUANG2, and Yaw-Huei LIN7 *
1Institute of Chinese Pharmaceutical Sciences, China Medical University, Taichung 404, Taiwan
2Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung 804, Taiwan
2Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung 804, Taiwan
3School of Pharmacy, College of Pharmacy, Nihon University, 7-7-1 Narashinodai, Funabashi, Chiba 274-8555, Japan
4Graduate Institute of Pharmaceutical Chemistry, College of Pharmacy, China Medical University, Taichung, Taiwan
5Graduate Institute of Pharmacognosy, Taipei Medical University, Taipei, Taiwan
6Department of Physiology, School of Medicine, China Medical University, Taichung 404, Taiwan
7Institute of Plant and Microbial Biology, Academia Sinica, Nankang, Taipei 115, Taiwan
4Graduate Institute of Pharmaceutical Chemistry, College of Pharmacy, China Medical University, Taichung, Taiwan
5Graduate Institute of Pharmacognosy, Taipei Medical University, Taipei, Taiwan
6Department of Physiology, School of Medicine, China Medical University, Taichung 404, Taiwan
7Institute of Plant and Microbial Biology, Academia Sinica, Nankang, Taipei 115, Taiwan
(Received December 1, 2009; Accepted February 8, 2010)
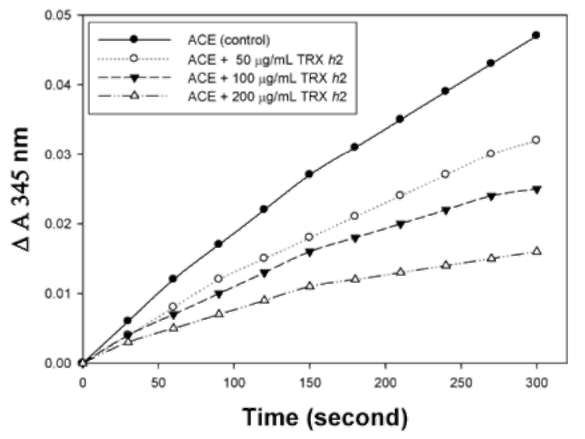
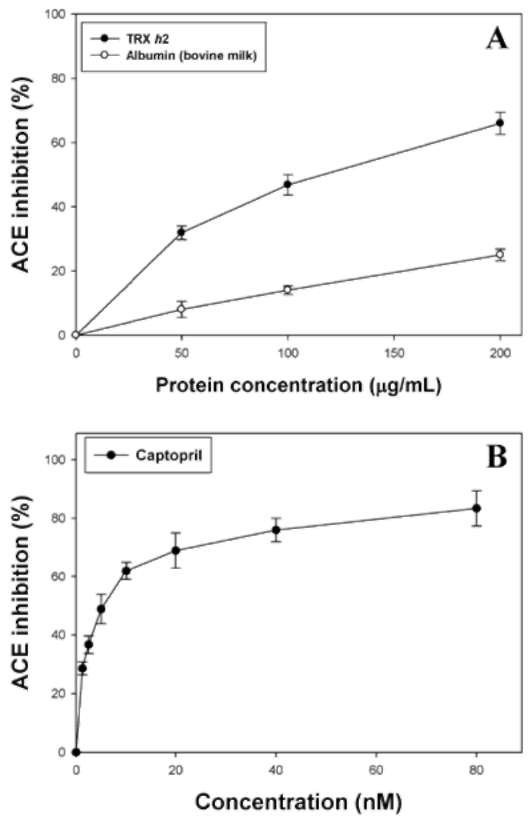
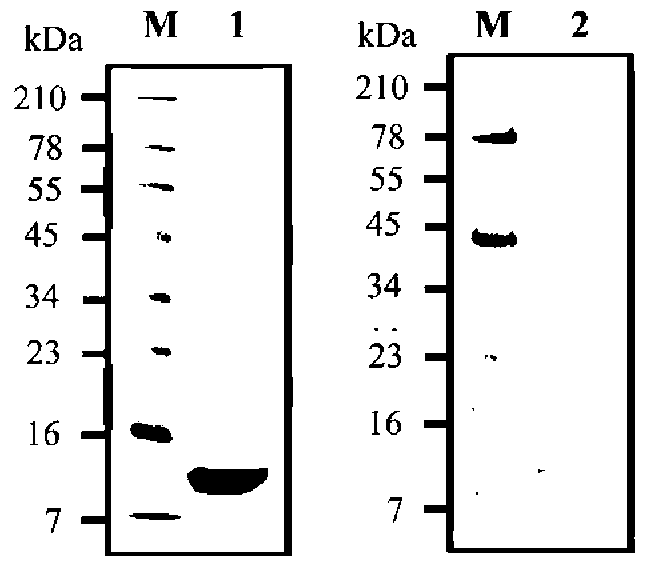
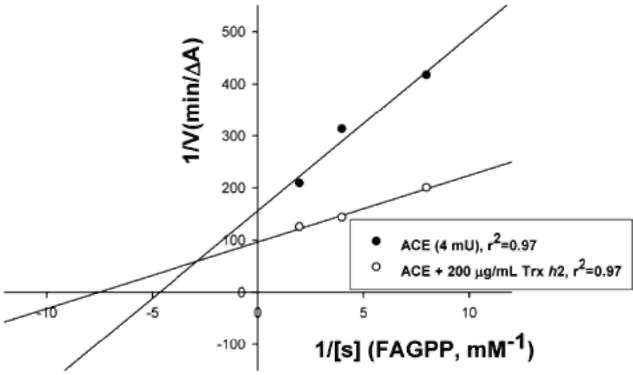
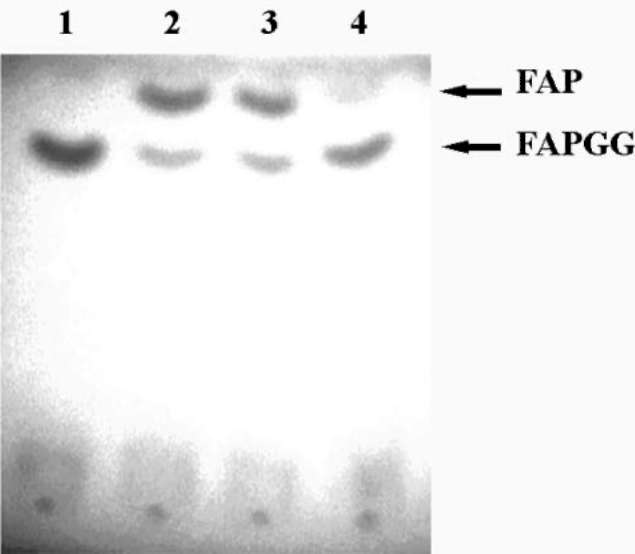
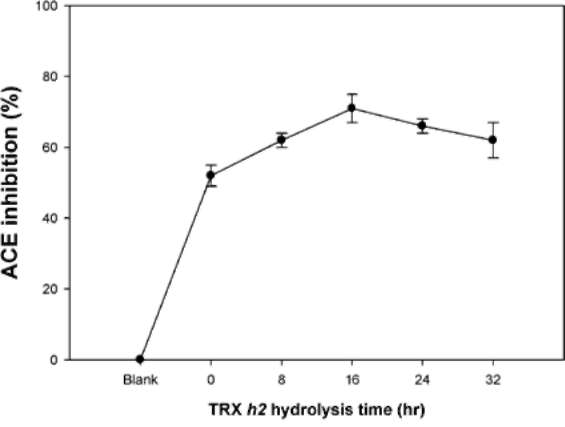
ABSTRACT. Recombinant thioredoxin h (Trx h2) overproduced in E. coli (M15) was purified by Ni2+-che-late affinity chromatography as previously reported (Huang et al., 2004a). The molecular mass of Trx h2 was ca. 14 kDa as determined by SDS (sodium dodecyl sulfate)-PAGE (polyacrylamide gel electrophoresis). Trx h2 had antioxidant (Huang et al., 2004b), dehydroascorbate reductase, and monodehydroascorbate reductase activities (Huang et al., 2008a). Trx h2 was shown by spectrophotometric methods to inhibit angiotensin converting enzyme (ACE) in a dose-dependent manner (50-200 (ig/mL, with 31.9 ~ 65.9% inhibition) using N-[3-(2-furyl) acryloyl]-Phe-Gly-Gly (FAPGG) as a substrate. A 50% inhibition (IC50) of ACE activity required 151.8 [ig/mL of Trx h2 and 10 nM (868 ng/mL) of Captopril. TLC data also showed Trx h2 as an ACE inhibitor. Trx h2 acted as a mixed type inhibitor against ACE using FAPGG as a substrate. When 200 [ig/mL Trx h2 were added, Vmax and Km were, respectively, 0.010 AA/min and 0.125 mM; without Trx h2 they were 0.0096 AA/min and 0.495 mM. Trypsin was used for Trx h2 hydrolysis over different time periods. ACE inhibitory activity was found to increase from 52% to about 72% after 16 h of hydrolysis. The results suggested that the ACE inhibitory capacity of small peptides increased through trypsin hydrolysis of Trx h2 up to 16 h and then decreased, which may have been due to the disappearance of some active ingredients. Four peptides, namely EVPK, VVGAK, FTDVDFIK and MMEPMVK, were synthesized based on the simulated trypsin digestion of Trx h2 and then tested for ACE inhibitory activity. The IC50 values of individual peptides were 1.73 ±0.24, 1.14 士 0.13, 0.42 ±0.02, and 1.03 ±0.58 mM, respectively, suggesting that FTDVDFIK might be the main active site of ACE inhibition.The results for Trx h2 and its hydrolysates might mean that consumption of sweet potato storage roots can aid in the control of hypertension and other diseases.
Keywords: Angiotensin converting enzyme (ace); Hydrolysis peptides; Sweet potato; Thioredoxin h2.
INTRODUCTION
Many bioactive peptides have common structural properties that include a relatively short peptide residue length (e.g. 2-9 amino acids) and the possession of hydropho-bic amino acid residues in addition to proline, lysine, or arginine groups. Bioactive peptides are among the many functional components identified in foods. These are small protein fragments that have biological effects once they
are released during gastrointestinal digestion in the organism or by previous in vitro protein hydrolysis. Bioactive peptides with immunostimulating (Fiat et al., 1993), anti-oxidant or angiotensin-converting enzyme inhibitor (Liu et al., 2007), antithrombotic (Scarborough et al., 1991), or bactericidal (Bellamy et al., 1993) functions have recently been the focus of research.
ACE (angiotensin converting enzyme, peptidyldipep-tide hydrolyase EC 3.4.15.1) is a glycoprotein and a dipep-tide-liberating exopeptidase classically associated with the renin-angiotensin system regulating peripheral blood pressure (Mullally et al., 1996; Lee et al., 2006). ACE removes a dipeptide from the C-terminus of angiotensin I to form
8These two authors contributed equally to this work. *Corresponding author: E-mail: boyhlin@gate.sinica.edu.tw; Fax: 886-2-2782-7954; Tel: 886-2-2789-9590 ext. 321.