Botanical Studies (2011) 52: 47-54.

Direct evidence of the symplastic pathway in the trap of the bladderwort Utricularia gibba L.
Tanya Chun-Chiao JUANG1, Sonya Di-Chiao JUANG2, and Zin-Huang LIU3 *
1Department of Life Science, National Taiwan University, Taipei 106, Taiwan 2Stanford University, Stanford, California 94305-4020, USA
3Department of Biological Sciences, National Sun Yat-Sen University, Kaohsiung 804, Taiwan
(Received November 27, 2009; Accepted June 10, 2010)
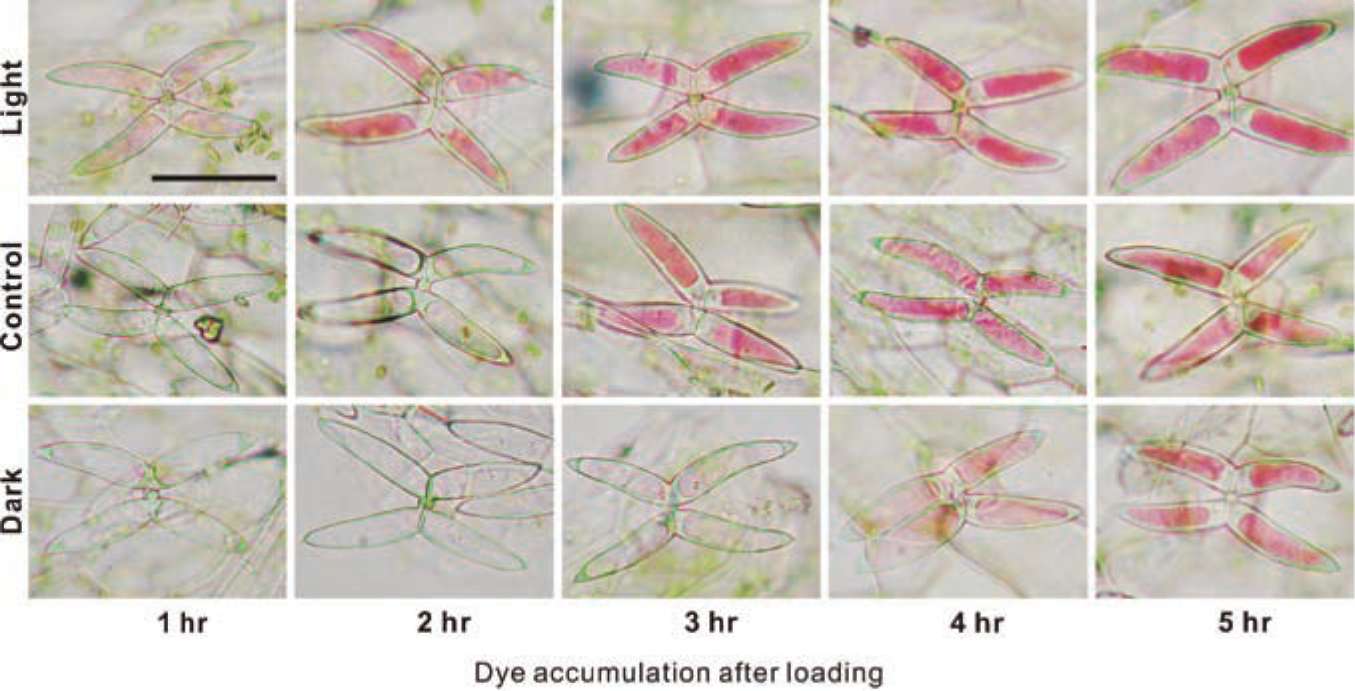
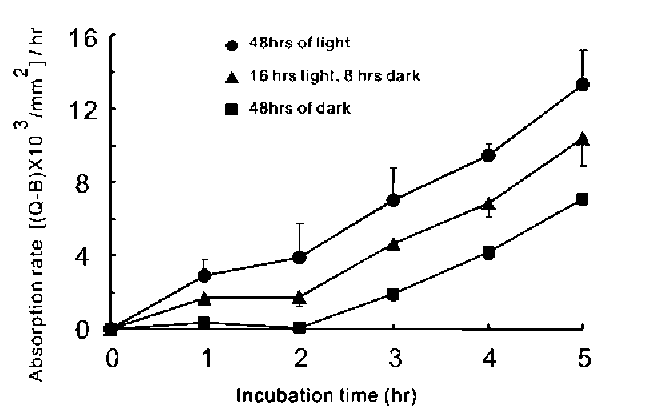
ABSTRACT. To capture prey, Utricularia gibba, an aquatic angiosperm carnivorous plant, is equipped with specialized bladders containing bifid/quadrifid glands for nutrient absorption. Several studies have focused on the nutrient absorption and subsequent transportation in the bladderworts; more specifically, the apoplastic pathway has been demonstrated as employing radioactive uranium salts. Nevertheless, the symplastic pathway has not been unambiguously demonstrated. Herein, we initially used food dyes as tracers to monitor the absorption processes by light microscope. We then confirmed the observed symplastic pathway using another vital tracer carboxyfluorescein diacetate (CFDA). The absorption and transportation of the CFDA inside the traps were observed using epifluorescence microscope and confocal laser scanning microscope. Our data clearly suggested that the tracers were transported through different tissues in the following order: terminal cells, pedestal cells, basal cells, surrounding epidermal cells, nearby leaves, and, finally, stems. The process was found to be light-sensitive, suggesting that it is energy-dependent. The uptake of the fluorescent dye was observed within seconds, while that of food dyes required 2 to 3 h. Thus, CFDA provides better resolution, while the food dyes afford prolonged tracing procedures. Taken together, the findings lead us to conclude that the symplastic pathway is an important transportation process that has never been shown previously in Utricu-laria.
Keywords: Carboxyfluorescein; Symplastic transport; Utricularia gibba.
INTRODUCTION
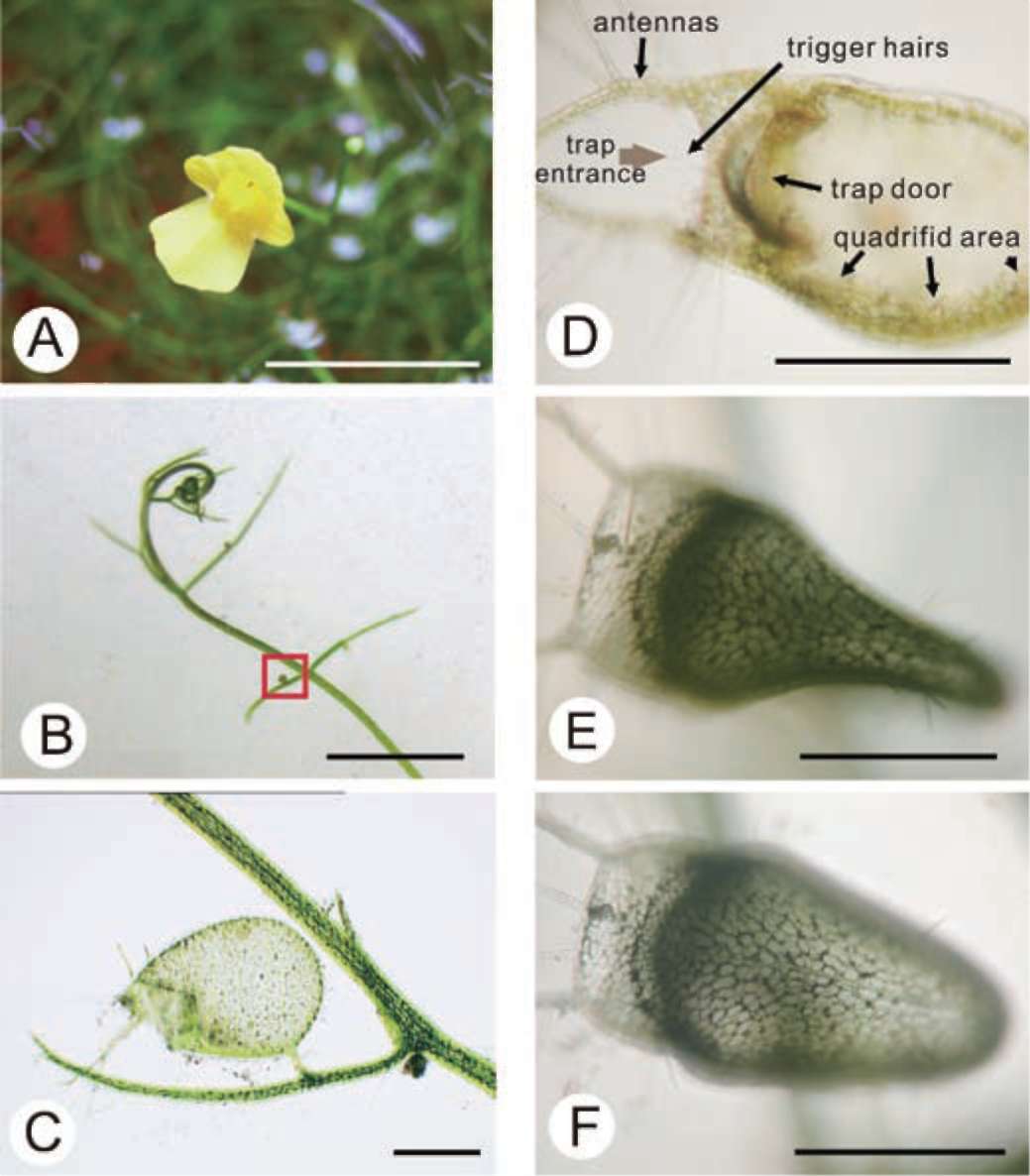
small water animal touches it, the threshold swings open inwardly, causing the water animal to be sucked into the trap along with rushing water. All of this happens in about 1/30 second (Figure 1D). Over a period of half an hour to an hour, the trap mechanism is automatically reset in preparation for the next catch and the pressure inside the trap is kept lower than the outside (Sydenham and Findlay, 1975). Because of this pressure difference, when the trap is viewed from above, the walls are warped inwards and appear concave (Figure 1E and 1F).
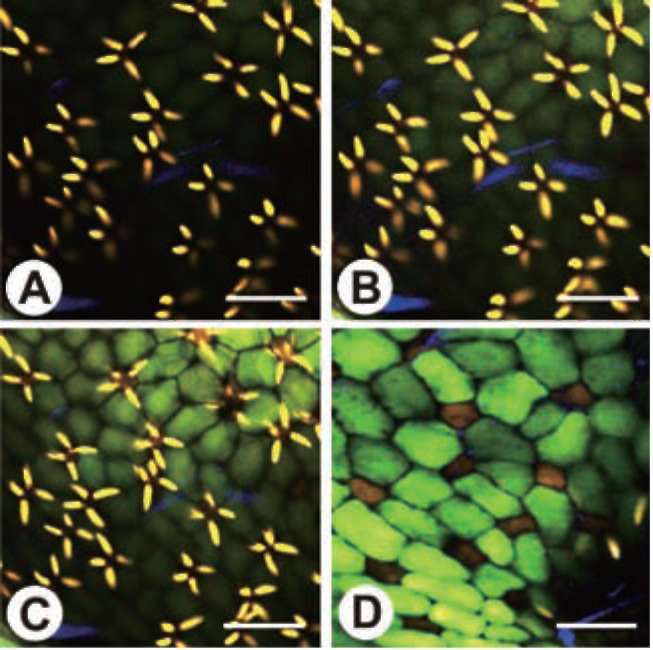
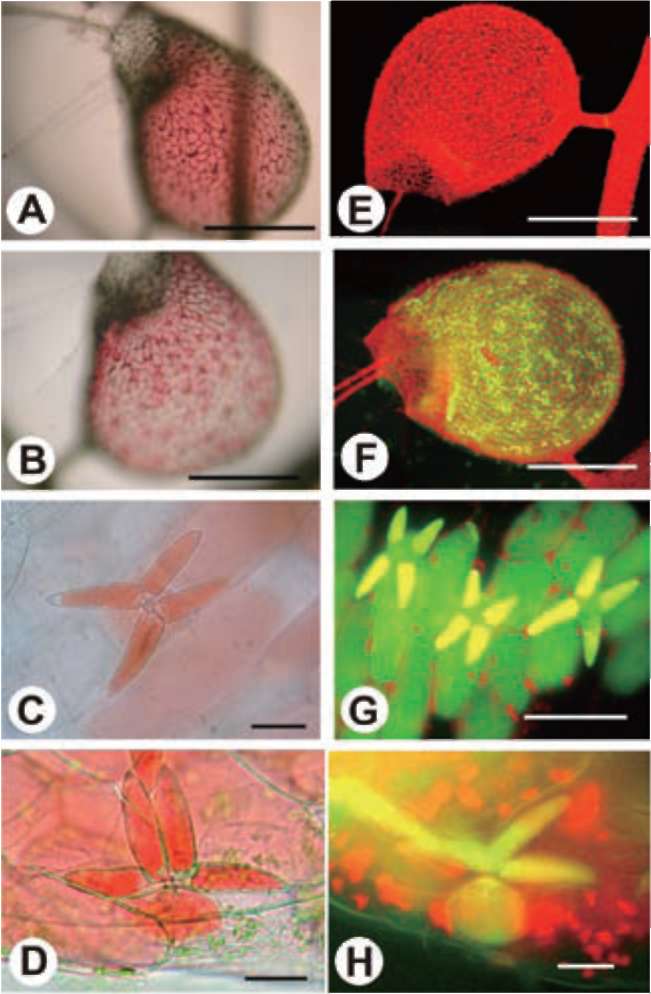
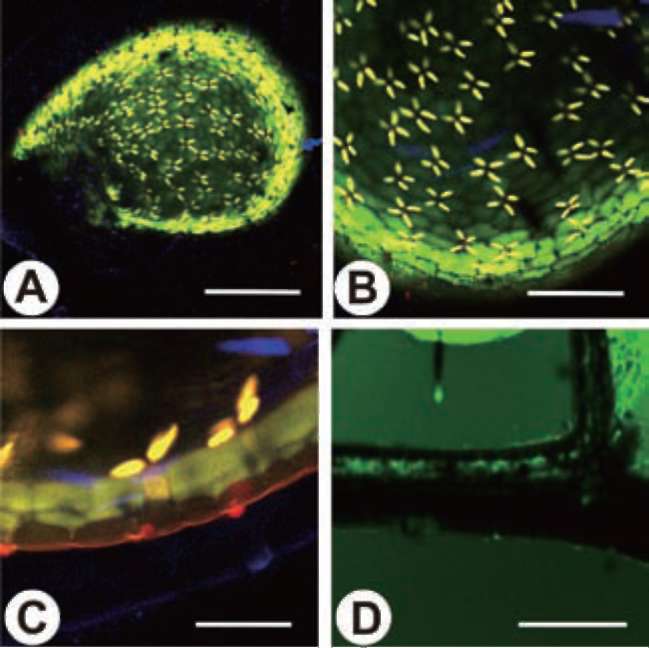
The inner surface of the trap is covered by quadrifid hairs. Only the inner surface of the threshold is covered by bifid hairs. Both quadrifids and bifids consist of a basal cell, a pedestal cell, and several terminal cells, which bear a complex architecture (Finerran, 1985). The pedestal cells of these glands have differentiated into transfer cells, and the cell walls are impregnated (Offler et al., 2003 ; Plachno and Jankun, 2004). The functions of the glands have been gradually clarified from the time of Darwin's early observations with a light microscope (1875) to recent studies based on the electron microscope. Their main functions include water removal from the lumen after prey-catching to maintain negative pressure inside the trap, transportation of nutrients, and the digestion and absorption of objects in-
Plants are considered the producers in the ecosystem, but carnivorous plants can derive nutrients from animals through carnivorous activities (Adamec, 1997). Starting with Darwin's Insectivorous Plants (1875), species of Utricularia have drawn the attention of many botanists. Utricularia gibba is herbaceous, angiospermic (Figure 1A), rootless, branching, with a green or brown stem 0.10.2 mm thick and up to 3 m long, with fine green branches bearing tiny bulbous traps, or bladders, and characteristic finely divided foliage (Figure 1B). A single trap is a small ovoid bladder up to 4 mm in length, with an entrance and a stalk that attaches it to the plant (Figure 1C). These sacs are highly sophisticated mechanical traps with a self-resetting mechanism capable of catching tiny water animals with amazing efficacy. Each trap has some antenna-like hairs on one side of the trap opposite the attaching stem. The ventral part of the trap wall in the entrance forms the threshold. There are trigger hairs on it, and when a
*Corresponding author: E-mail: zhliu@mail.nsysu.edu.tw; Tel: 886-7-5252000 ext. 3617 Fax: 886-7-525-3609.