Botanical Studies (2011) 52: 55-72.

The XopE2 effector protein of Xanthomonas campestris pv. vesicatoria is involved in virulence and in the suppression of the hypersensitive response
Rong-Hwa LIN1'3'4, Chih-Wen PENG2, Yuan-Chuen LIN3, Hwei-Ling PENG4, and Hsiou-Chen HUANG3*
1Biotechnology Center, National Chung Hsing University, Taichung, 40227, Taiwan
2Department of Life Science, Tzu-Chi University, Hualien 97004, Taiwan
3Graduate Institute of Biotechnology, National Chung Hsing University, Taichung, 40227, Taiwan
4Department of Biological Science and Technology, National Chiao Tung University, Hsin Chiu 30050, Taiwan
2Department of Life Science, Tzu-Chi University, Hualien 97004, Taiwan
3Graduate Institute of Biotechnology, National Chung Hsing University, Taichung, 40227, Taiwan
4Department of Biological Science and Technology, National Chiao Tung University, Hsin Chiu 30050, Taiwan
(Received May 14, 2010; Accepted June 29, 2010)
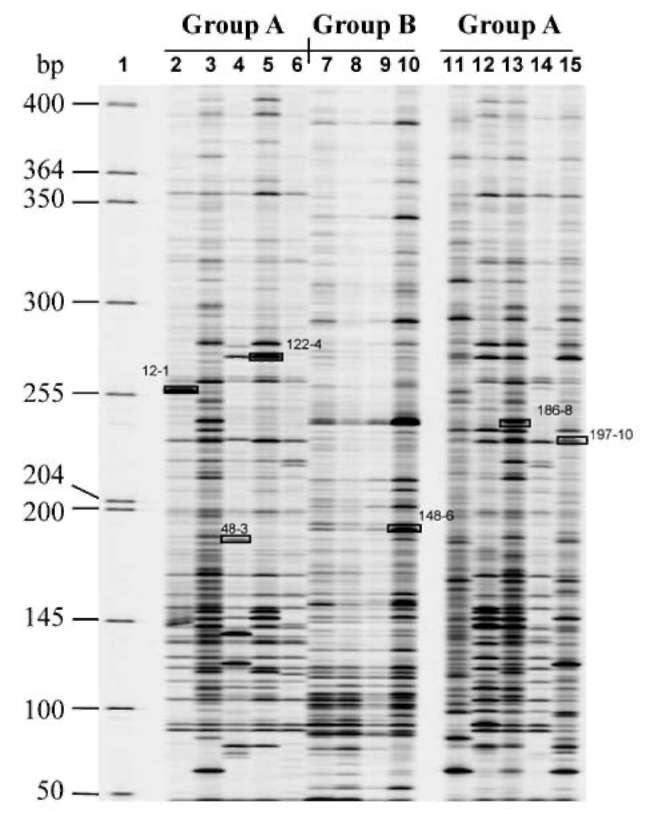
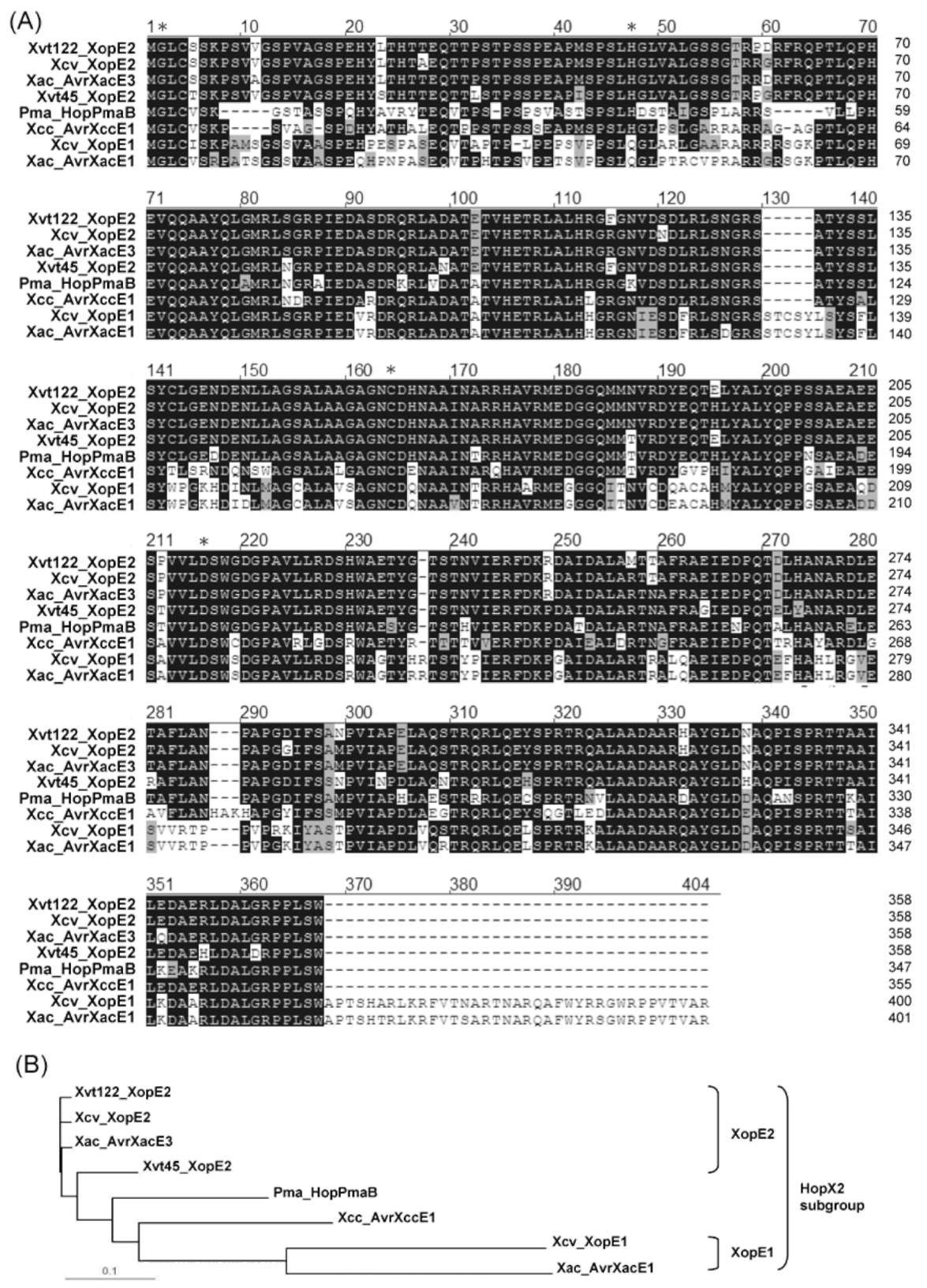
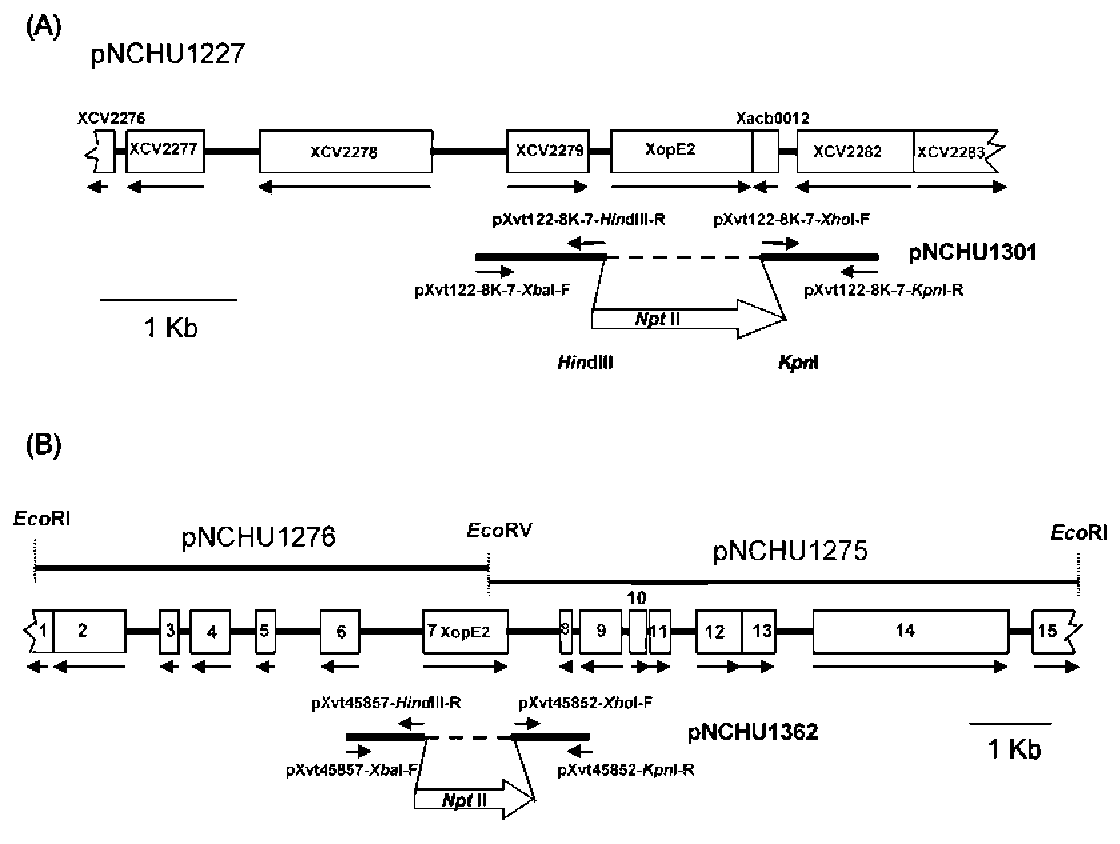
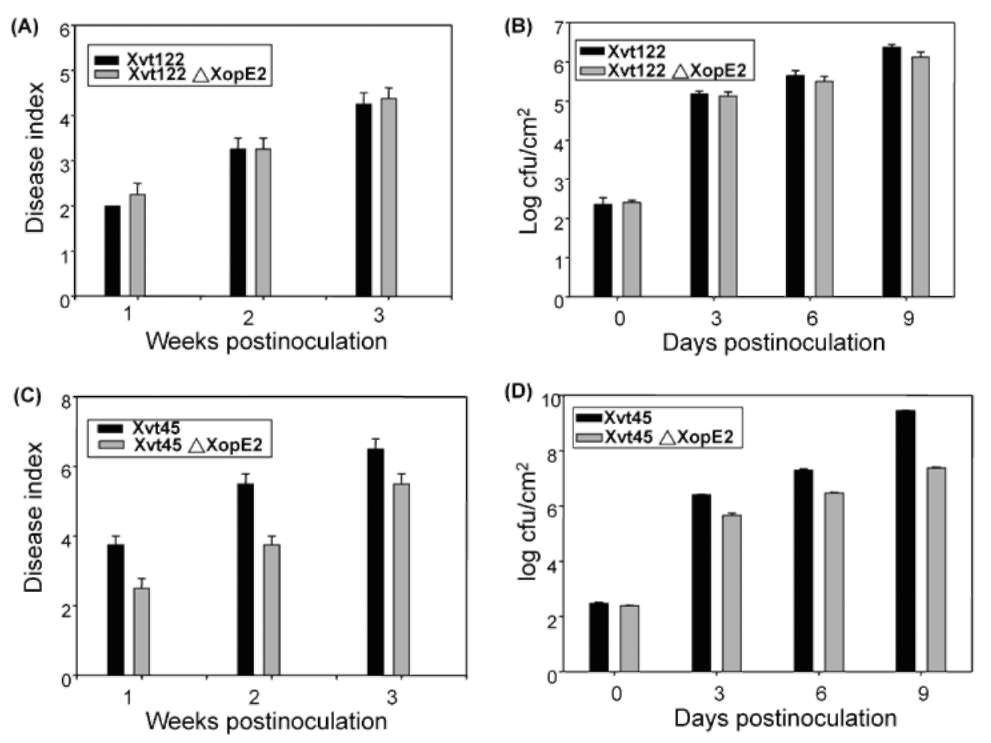
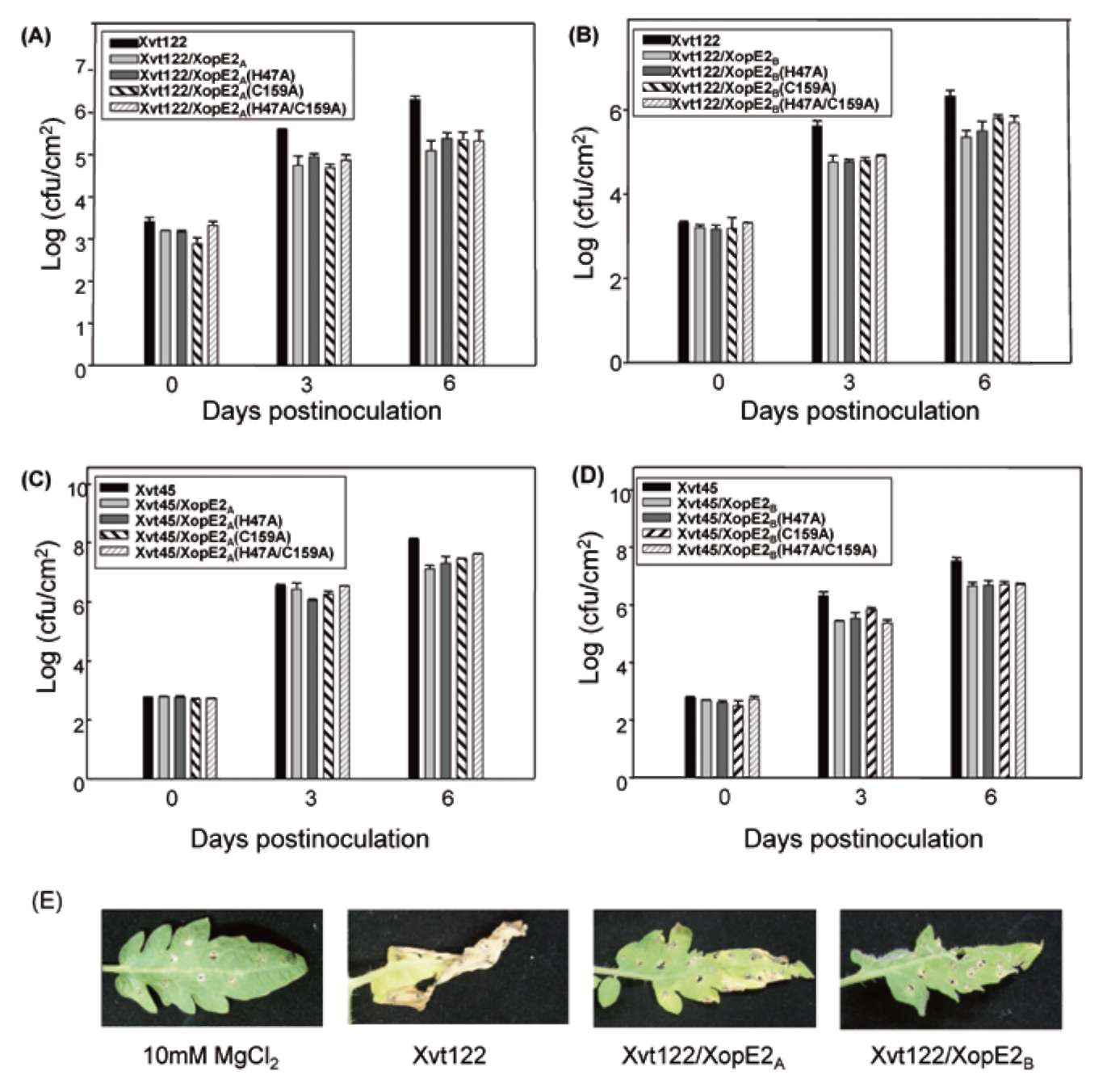
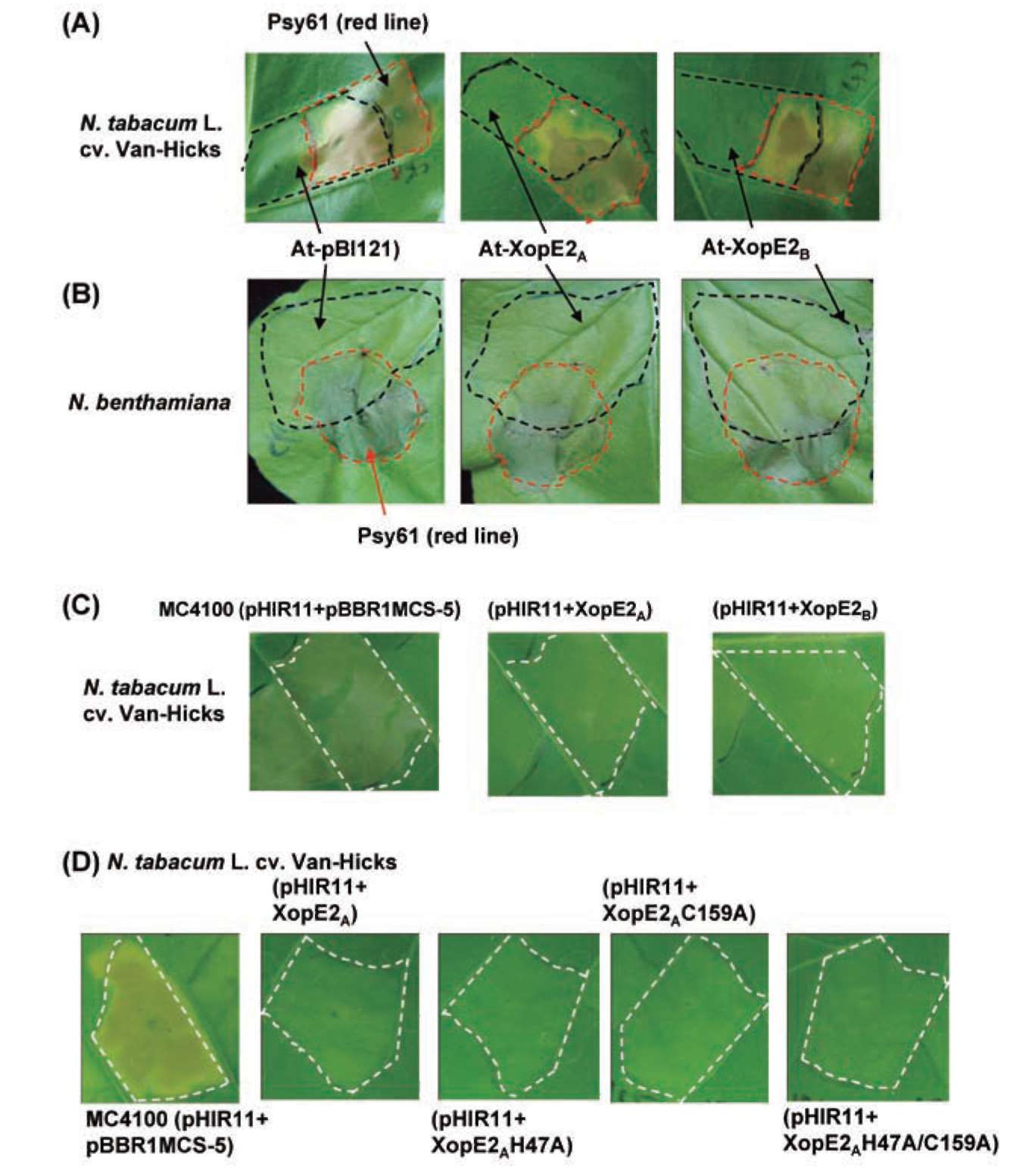
ABSTRACT. Pathogenicity of Xanthomonas campestris pv. vesicatoria (Xcv) causing bacterial spot disease on tomato (Lycopersicon spp.) and pepper (Capsicum spp.) requires the type III secretion system (T3SS) and T3SS effectors. In this study, we employed the AFLP technique to investigate the diversity of X. campestris pv. vesicatoria isolated in Taiwan, and consequently a XopE2 homologue was identified in all fourteen Xcv strains that have been classified into two groups. Phylogenic analysis of XopE2 amino acid sequences indicated that XopE2 of Xcv Xvt122 (group A) has a closer genetic distance to XopE2 of Xcv 85-10 than to that of Xcv Xvt45 (group B). Interestingly, although it was suggested that Xvt45 contains duplicated xopE2 genes, one being located on the genome and the other located on a large plasmid, a single copy deletion of xopE2 within the genome caused a substantial reduction in virulence, but no effect of xopE2 mutation on virulence of Xcv 85-10 and Xvt122 was observed. Furthermore, our results revealed that XopE2 of Xcv Xvt122 or Xcv Xvt45 was able to suppress HR in a T3SS-dependent manner and the heterologously-expressed XopE2 was sufficient to modulate the virulence on susceptible tomato plants. Their biological functions are not dependent on the consensus catalytic triad (159th cysteine) and thiol-protease His residue (47th histidine) of XopE2.
Keywords: Amplified restriction fragment length polymorphism; Bacterial spot; Hypersensitive response; Type III secretion system; XopE2 effector.
Abbreviations: AFLP, Amplified restriction fragment length polymorphism; T3SS, type III secretion system; HR, hypersensitive response; avr, avirulence; PCD, programmed cell-death.
INTRODUCTION
nicity)/ hrc (hypersensitive response and conserved) gene clusters. The T3S machinery secretes proteins into the extracellular milieu (e.g. harpin or pilus proteins) and translocates effector proteins (e.g. Xop or Avr proteins) into the plant cell (Grant et al., 2006; Gurlebeck et al., 2006; Kay and Bonas, 2009). Some effectors designated as avirulence (avr) proteins are specifically recognized in resistant plants containing corresponding resistance (R) genes, usually resulting in a hypersensitive response (HR) that restricts bacterial growth via programmed cell-death (PCD) reactions (Klement, 1982; Staskawicz et al., 2001; Grant et al., 2006; Mudgett, 2005). The T3S mutants that are impaired in growth in planta also fail to cause disease symptoms in susceptible plants and lose the capacity to induce the HR in non-hosts or resistant hosts, indicating that T3S effectors may play essential roles in the interaction of bacteria with plants (Kay and Bonus, 2009).
Plants are armed with an elaborate network of defense mechanisms to protect themselves from the invasion of microorganisms. On the other hand, bacterial pathogens have evolved sophisticated strategies to conquer their plant hosts, i.e., they suppress the basic defense mechanisms in order to successfully establish the initiation of invasion (Nurnberger et al., 2004; Schechter et al., 2006). Similar to most Gram-negative phytopathogenic bacteria, Xanthomanas sp. possesses a conserved type III secretion (T3S) system which contains a needle-like structure and is encoded by hrp (hypersensitive response and pathoge-
*Corresponding author: E-mail: hchuang@dragon.nchu.edu. tw; Telephone: 886-4-22852155; Fax: 886-4-22853527.