Botanical Studies (2011) 52: 73-78.

Programmed cell death induced by heat shock in mung bean seedlings
Valentina P. EGOROVA1,2, Yih-Shan LO1, and Hwa DAI1*
(Received June 4, 2010; Accepted July 13, 2010)
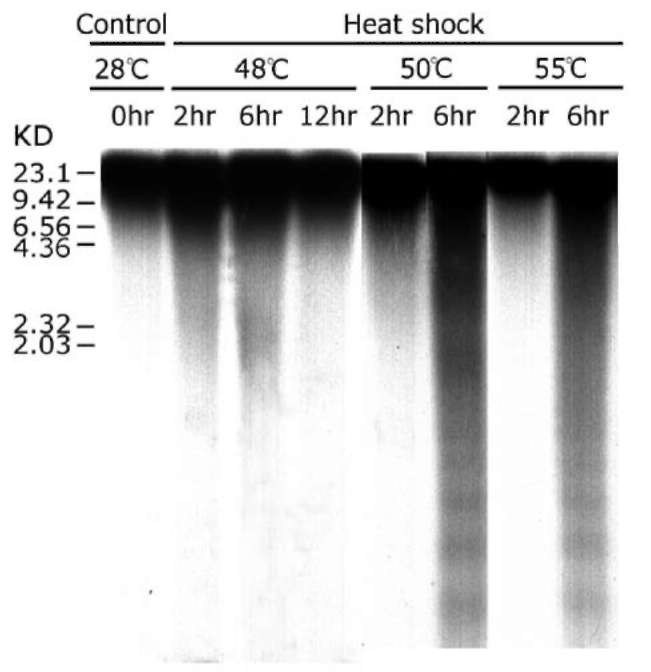
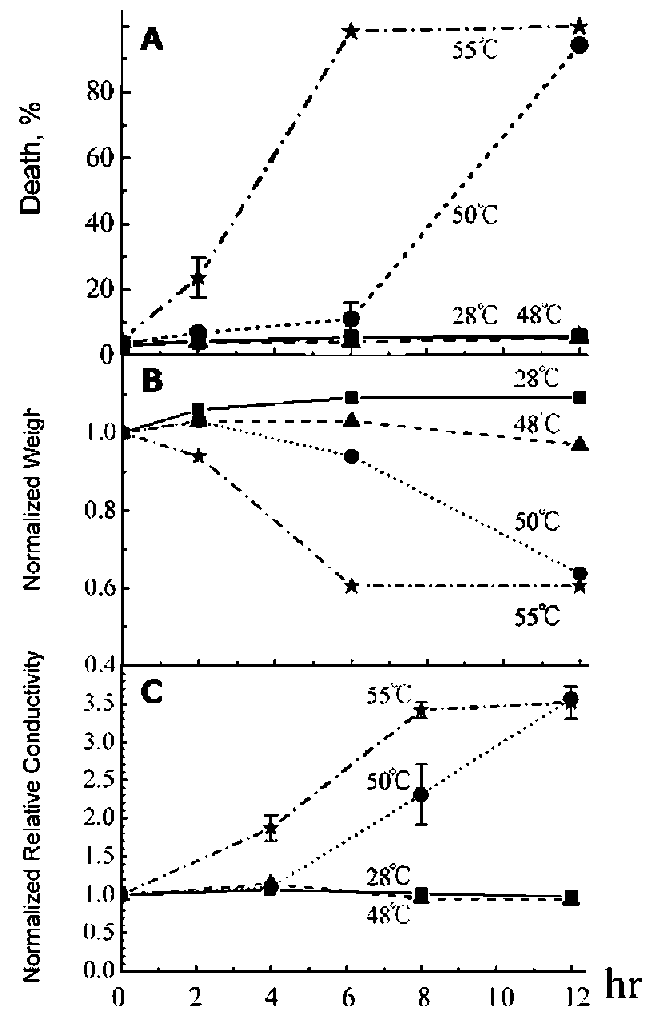
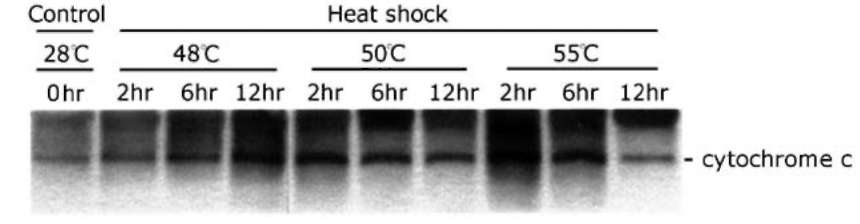
ABSTRACT. The effect of heat shock (HS) on death, weight and membrane leakage of mung bean seedlings as well as on early biochemical markers of PCD (cytochrome c release from mitochondria and internu-cleosomal DNA fragmentation) was studied. It was found that heat shock ranging from 48-55。C stimulated the releasing of cytochrome c from mitochondria to cytosol and the nuclear DNA fragmentation occurred during the first six hours of heat treatment. Sublethal heat shock (48°C) gives rise to non-ladder DNA degradation. Lethal heat shock (50 and 55。C) generates two types, ladder and non-ladder, DNA degradation. Internu-cleosomal (ladder) DNA fragmentation and cytochrome c releasing suggest the programmed cell death occurs in mung bean seedling induced by heat shock.
Keywords: Cytochrome c; DNA fragmentation; Heat shock; Programmed cell death.
INTRODUCTION
nucleosomal DNA degradation has been observed during certain development events (Young and Gallie, 2000; He and Kermode, 2003), after induction by different stresses (Koukalova et al., 1997), and during pathogen-induced death (Mittler and Lam, 1997). DNA is first cleaved into large fragments of about 300 and/or 50 kb (Oberhammer et al., 1993), and these are further digested between nu-cleosomes resulting in DNA fragments that are multimers of about 180 bp monomers (Lagarkova et al., 1995). It is currently believed that these changes in genomic DNA are incompatible with cell survival and mark the point of no return in the execution stage of the PCD pathway.
In this work, we studied the effect of heat shock (HS) on death, weight and membrane leakage of mung bean seedlings as well as on early biochemical markers of PCD (cytochrome c release in cytosol and DNA fragmentation). It was found that all temperatures applied for heat shock (48-55°C) increase the releasing of cytochrome c from mitochondria to cytosol and causes a concomitant nuclear DNA fragmentation during the first six hours of heat shock at 50°C and 55°C. The sublethal heat shock (48。C) gave rise to non-ladder DNA fragmentation and lethal heat shock (50。C and 55。C) exhibited both ladder and non-ladder DNA degradation.
Programmed cell death (PCD) occurred during plant developmental processes such as flower development, embryogenesis, seed germination, and vessel and trachea formation was reported before (Pennell and Lamb, 1997; Mittler, 1998; Egorova et al., 2010). In addition, PCD can be also triggered in plant cells infected by pathogens, wound by physical treatments or damaged by low dosage of toxic compounds (Buckner et al., 2000).
One of the earliest markers of animal PCD is the releasing of cytochrome c from mitochondria to cytosol (Reed, 1997; Reape and McCabe, 2010). By analogy with mammalian apoptosis, plant mitochondria have been suggested to play a pivotal role in the integration of environmental and developmental signals that trigger cell death (Lam et al., 2001). Few studies, however, have explored the possible involvement of cytochrome c and mitochondria in plant PCD (Balk et al., 1999; Balk and Leaver, 2001; Yu et al., 2002; Reape and McCabe, 2010). Nuclear DNA
degradation, which occurs following the initial stage of chromatin condensation, is an early feature of both animal and plant PCD (Krishnamurthy et al., 2000). The orderly degradation of genome during PCD is contrast to random decay of DNA that follows other types of cell death such as injury-induced necrosis (Kerr et al., 1995). In plants,
MATERIALS AND METHODS
Plant material
2Present address: Institute of Bioorganic Chemistry, Belarus National Academy of Sciences, Kuprevich St. 5/2, 220141 Minsk, Belarus.
*Corresponding author: E-mail: bodaihwa@gate.sinica.edu. tw; Tel: 886-2-27871176; Fax: 886-2-27838609.
Mung bean seeds (Vigna radiata L. (Wilczek) cv. Tainan) were grown as previously described (Dai et al., 1998). To induce HS, three-day-old seedlings were incubated for 12 h in a dark growth chamber (humidity 67%) at 48°C, 50。C, and 55。C (Altschuler and Mascarenhas, 1982). Con-