Botanical Studies (2011) 52: 99-104.

Ypsilandra (Melanthiaceae; Liliaceae sensu lato), a new generic record for Taiwan
Tsai-Wen HSU1,2, Yoshiko KONO3, Tzen-Yuh CHIANG2, and Ching-I PENG3 *
lTaiwan Endemic Species Research Institute, Chichi, Nantou 552, Taiwan
2Department of Life Sciences, National Cheng-Kung University, Tainan 701, Taiwan
3Biodiversity Research Center, Academia Sinica, Nangang, Taipei 115, Taiwan
2Department of Life Sciences, National Cheng-Kung University, Tainan 701, Taiwan
3Biodiversity Research Center, Academia Sinica, Nangang, Taipei 115, Taiwan
(Received April 8, 2009; Accepted June 9, 2010)
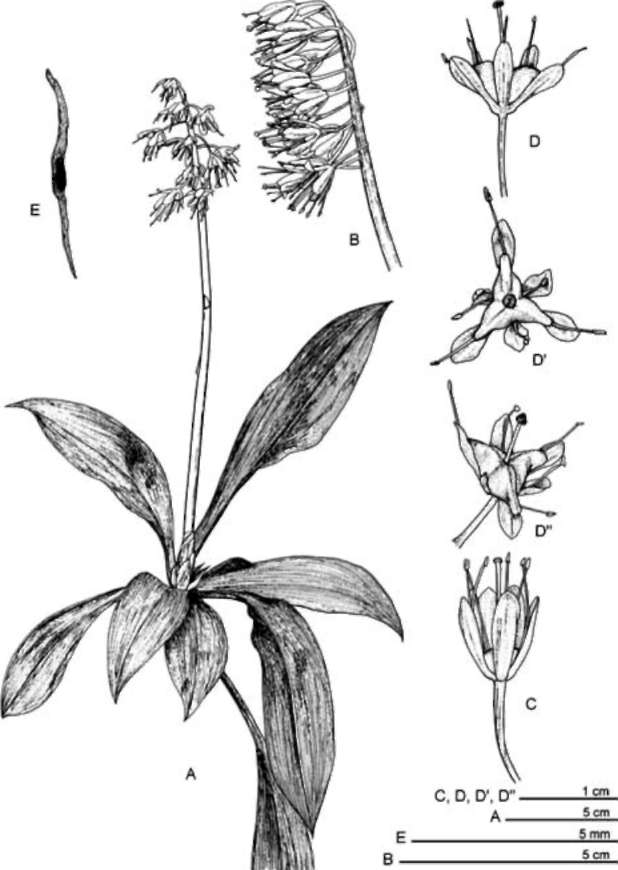
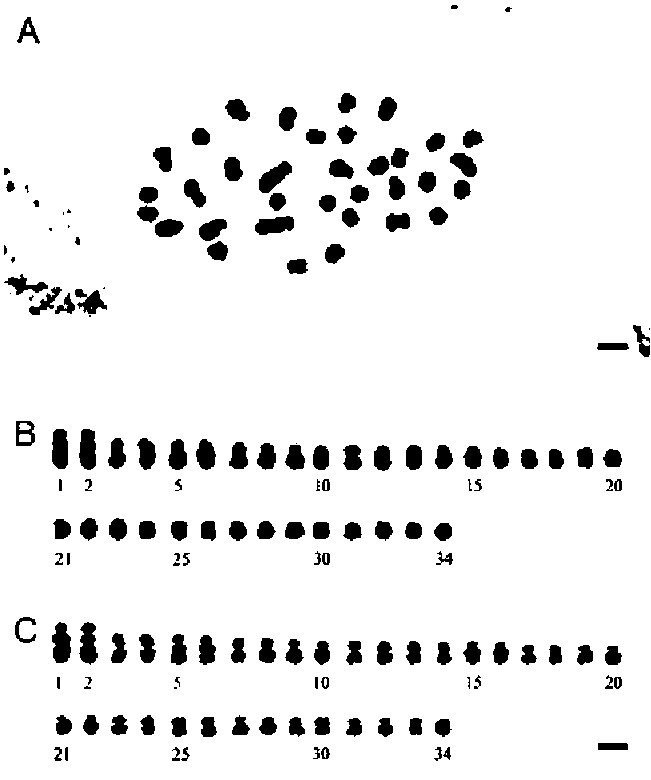
ABSTRACT. Ypsilandra thibetica Franch. (Liliaceae sensu lato), previously known only from southcentral and southern China (Sichuan, S. Hunan and NE Guangxi), was recently found in mountainous regions of central and eastern Taiwan. The discovery adds a new record for the species, and for the genus, to the flora of Taiwan. Ypsilandra can be easily distinguished from other genera of Liliaceae by its rosette of basal leaves, racemose inflorescence, and 3-lobed capsules. This paper provides a taxonomic account of the genus, a line drawing and color photographs to aid in identification. The somatic chromosome number and karyotype formula (2n = 34 = 18m+10sm4SC+6st2SC ) are reported for Y thibetica for the first time.
Keywords: Chromosome cytology; Karyotype; Liliaceae; Melanthiaceae; Taiwan; Taxonomy; Ypsilandra thibetica.
INTRODUCTION
ing of chromosomes for observations follow Oginuma and Nakata (1988). Classification of chromosome morphology is based on the position of the centromere (Levan et al., 1964).
Taxonomic Treatment
Ypsilandra Franch., Nouv. Arch. Mus. Hist. Nat. Paris, Ser. 2, 10: 93. /.77.1887. 丫蕊花屬
Herbs, perennial, glabrous. Rhizome stout, short. Leaves basal, in a rosette, linear to oblanceolate or spat-ulate-oblanceolate. Peduncle erect, simple, with several to many sheathing or bract-like leaves. Inflorescence scapiform, a terminal raceme, 4-30-flowered, ebracteate except in Ypsilandra^jinpingensis (Chen et al., 2003). Flowers bisexual, actinomorphic. Tepals 6, persistent, with a basal nectary gland on adaxial surface. Stamens 6, free from tepals, exceeding or nearly as long as tepals; anthers reniform, basifixed, thecae confluent. Ovary superior, 3-loculed, placentation axile; ovules many per locule; style 1, stigma capitate to 3-cleft. Capsules 3-lobed apically, trigonous, loculicidal; seeds numerous, fusiform to linear, tailed at both ends.
Six species (Ypsilandra alpina F. T. Wang & T. Tang, Y. cavaleriei H. Lev. & Vaniot., Y. jinpingensis W. H. Chen, Y. M. Shui et Z. Y. Yu, Y kansuensis R.N. Zhao & Z.X. Peng, Y thibetica Franch., Y yunnanensis W.W. Smith & Jeffrey); Bhutan, China, Myanmar, Nepal, Vietnam (Chen and Tamura, 2000; Shaw, 2008); one species in Taiwan (reported here).
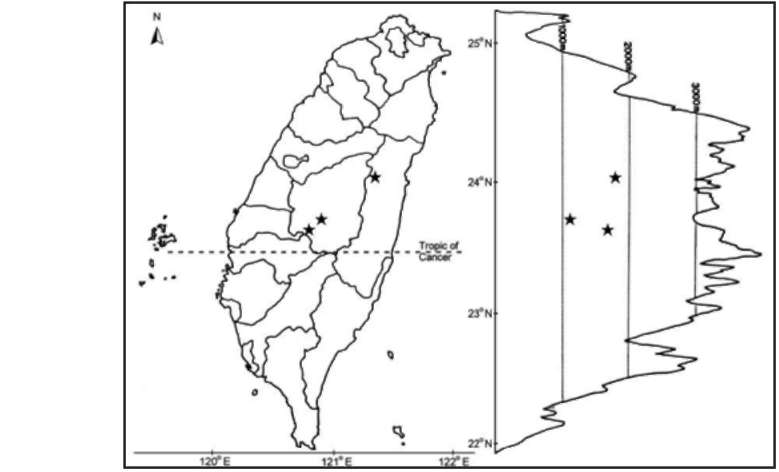
Liliaceae sensu lato, a family widespread throughout the world, consists of about 280 genera and 4,000 species (Cronquist, 1981). Taxonomy of the family in Taiwan has been revised recently by Ying (1988, 1989, 1990), Huang and Yang (1988), Hara (1987, 1988), Tanaka (1998, 2001a, 2001b), Lang et al. (1999), Ohashi (2000), Hiramatsu et al. (2001) and Peng et al. (2007). Twenty-two genera and 31 species, one subspecies and fourteen varieties were record-ed in the Flora of Taiwan, 2nd Edition (Ying, 2000). During our botanical inventory of Taiwan, Ypsilandra thibetica Franch., a heretofore unrecorded species, was discovered in mountainous regions in central and eastern Taiwan. Yp-silandra Franch. comprises six species in mainland China, Bhutan, Myanmar, Nepal, and Vietnam (Chen and Tamura, 2000; Chen et al., 2003; Shaw, 2008). Our discovery
of Y. thibetica on Taiwan represents a significant range extension for both the genus and the species.
MATERIALS AND METHODS
Plants of Ypsilandra thibetica from Nantou Hsien, Taiwan were cultivated in the experimental greenhouse of Academia Sinica, Nankang, Taipei, Taiwan. Somatic chromosomes were observed using at least three cells per individual. Methods of pretreatment, fixation, and stain-
*Corresponding author: E-mail: bopeng@sinica.edu.tw.