Botanical Studies (2011) 52: 121-127.

Polystichum cavernicola, sp. nov. (sect. Haplopolysti-chum, Dryopteridaceae) from a karst cave in Guizhou, China and its phylogenetic affinities
Hai HE1 and Li-Bing ZHANG2 *
1Department of Biology, Chongqing Normal University, Shapingba, Chongqing 400047, China
2 Chengdu Institute of Biology, Chinese Academy of SSciences, P.O. Box 416, Chengdu, Sichuan 610041, China and Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, USA
(Received January 25, 2010; Accepted August 10, 2010)
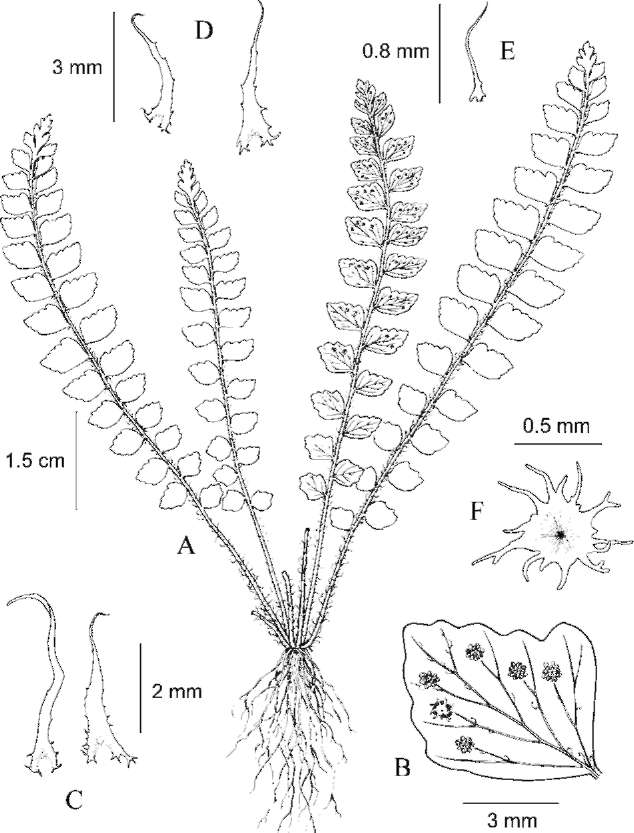
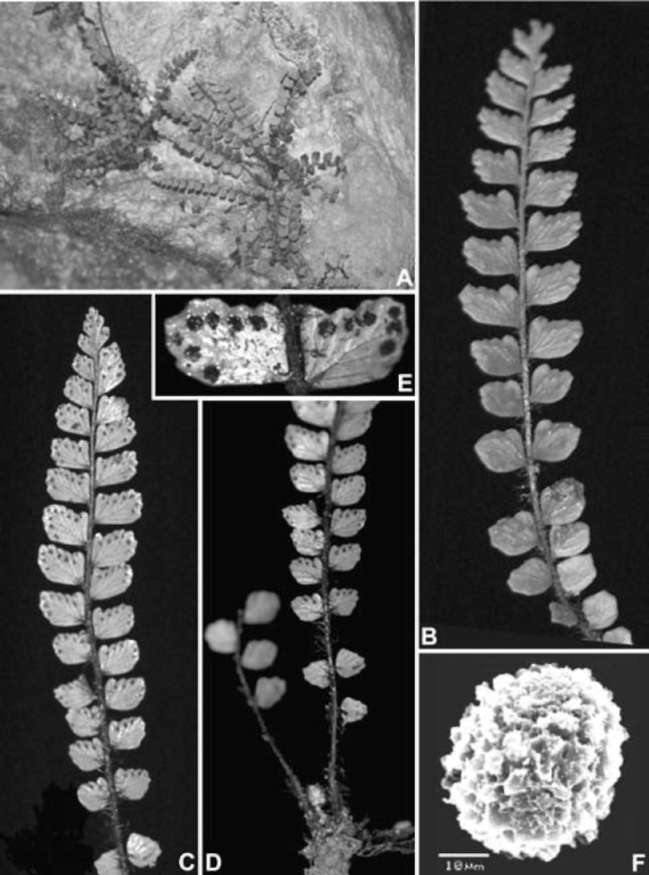
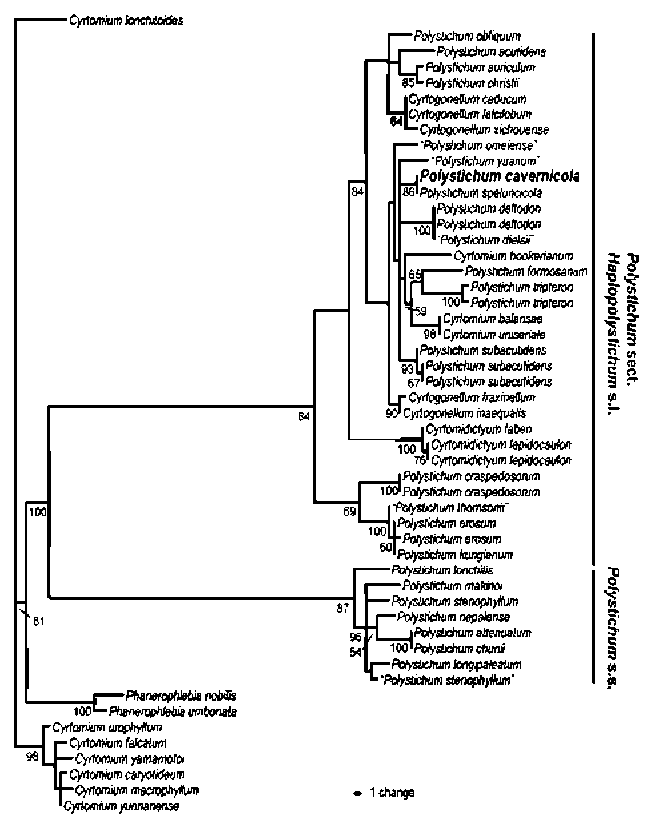
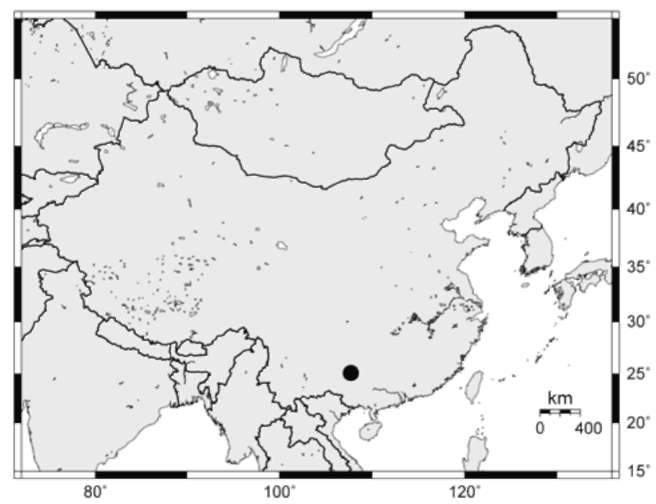
ABSTRACT. Polystichum cavernicola L. B. Zhang & H. He, a new pteridophyte species is described and illustrated from a karst cave in southern Guizhou, China. It is a member of Polystichum sect. Haplopolystichum (Dryopteridaceae). A phylogenetic analysis based on the chloroplast trnL-F sequences shows that the new species is most closely related with P. speluncicola, a species also described from a karst cave in southern Guizhou. Morphologically, P. cavernicola is most similar to P. speluncicola. The important morphological differences between P. cavernicola and P. speluncicola include that P. cavernicola has narrow-type microscales on the abaxial laminar surface, its pinnae are chartaceous and have auriculate acroscopic bases, and its lamina is broadest near the midpoint, whereas P. speluncicola has broad-type microscales on the abaxial laminar surface, its pinnae are subcoriaceous and have rounded, non-auriculate, acroscopic bases, and its lamina is broadest above the middle. The spores of P. cavernicola have verrucate sculpturing on the perispore, whereas those of P. speluncicola are cristate with numerous spinules on its perispore. Polystichum cavernicola is endemic to a single karst cave in southern Guizhou and is considered to be Critically Endangered (CR) based on IUCN Red List criteria.
Keywords: Cave flora; Fern; Dryopteridaceae; Guizhou; Phylogeny; Polystichum cavernicola; sect. Hap-lopolystichum; Spore morphology; TrnL-F sequence.
INTRODUCTION
CHINA. Guizhou: Libo County, Wong'ang Town, Jilong Village, 4 Nov 2008, L. B. Zhang, H. He & C. B. Jiang 911 (CDBI, CTC, MO, Herb. Pei-Shan Wang).
Morphological study. The measurement of roots, petioles, rachises, scales, and indusia was conducted with a micrometer under a dissecting microscope.
Molecular methods. Total genomic DNA was isolated from silica-dried leaves using Plant Genomic DNA Kits
(TIANGEN BioTech., Beijing, China). The plastid trnL-F
intergenic spacer was amplified using the universal primers e and f of Taberlet et al. (1991). The PCR protocols followed Zhang et al. (2001). Amplified fragments were purified with TIANquick Mini Purification Kits (TIAN-GEN). Purified PCR products were sequenced by Invitro-genTM (Shanghai, China).
Based on previous phylogenetic analyses (Driscoll and Barrington, 2007; Lu et al., 2007; Li et al., 2008; Zhang and He, 2010; Zhang et al., 2010), we included in the in-group 27 species of Polystichum sect. Haplopolystichum Tagawa sensu lato (s.l.; Zhang and He, 2009a) including sects. Crucifilix Tagawa, Haplopolystichum, and Spha-enopolystichum Ching ex W. M. Chu & Z. R. He, and genera Cyrtogonellum Ching and Cyrtomidictyum Ching,
During field work in 2008 we collected a few specimens and DNA samples of an undertermined species of Polystichum Roth (Dryopteridaceae) sect. Haplopolys-tichum Tagawa in a karst cave in Libo County, southern Guizhou, China. Like many of the species in sect. Hap-lopolystichum, it has a limited number of morphological characters available to infer its taxonomic identity and phylogenetic affinities. We therefore supplemented our macromorphological and palynological studies with a molecular analysis based on DNA sequences of the chlo-roplast trnL-F intergenic spacer region. We conclude that our collections represent an undescribed species, which we describe here.
MATERIAL AND METHODS
Materials examined. The morphological, palynological, and molecular data were based on the voucher specimens:
*Corresponding author: E-mail: Libing.Zhang@mobot.org; Tel: +1-314-577-9454; Fax: +1-314-577-9596.