LI et al. — mtDNA replication and transcription are affected by associated mitochondrial membrane
139
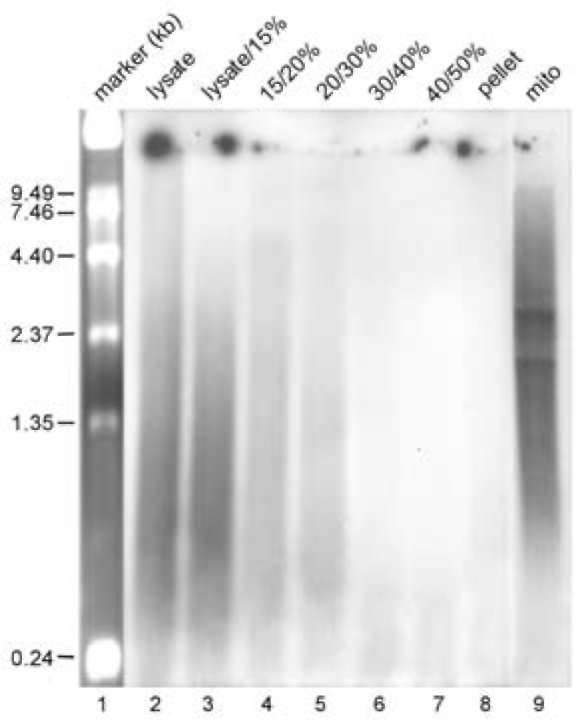
centrifugation at 46,000 x g for 1-2 h to pellet the mito-chondrial nucleoid-membrane complexes. PFGE analysis was carried out as described above. The X-ray film was developed after drying the gel.
Mitochondrial nucleoid-membrane complex transcription in vitro
In vitro mitochondrial nucleoid-membrane complex transcription was adapted with some modifications from a mitochondrial RNA synthesis method (Martin et al., 1987; Dai et al., 2005). Seven mitochondrial nucleoid-membrane complex fractions prepared from 4 mg protein equivalent mitochondria as described in the above section were suspended in 300 μl reaction buffer including 10 mM Tris-base (pH 8.5), 5 mM MgCl2, sucrose 0.25 M, 1 mM DTT, base (pH 8.5), 5 mM MgCl2, sucrose 0.25 M, 1 mM DTT,0.1% BSA, 120 mM each of CTP, GTP and ATP, 90 μCi α-32P-UTP and 100 units RNAsin, and then incubated at 25°C for 30 min before adding unlabeled UTP (final con-centration: 20 μM) continuing to incubate at 25°C for 5 min. After stopping the reaction by adding SDS and CDTA to final concentrations of 1% and 30 mM, respectively, newly synthesized RNA was isolated and purified using phenol/chloroform/isoamyl alcohol. The RNA was ana-lyzed with a standard MOPS gel containing formaldehyde (Dai et al., 2005). The newly-synthesized mitochondrial RNA was counted as described above.
RESULTS
The composition and amounts of phospholip-ids of mitochondrial nucleoid-membrane complexes derived from different sucrose gradient fractions
The phospholipid composition of mitochondrial nucle-oid-membrane complexes derived from each one of the sucrose gradient fractions remained similar to that of the whole mitochondria (Figure 1). However, the amounts of phospholipids of mitochondrial nucleoid-membrane complexes varied from one sucrose gradient fraction to another (Figure 1). In general, the amounts of phospholipids of mi-tochondrial nucleoid-membrane complexes decreased with the increase of banding density of mitochondrial nucleoid-membrane complexes (Figure 1), suggesting that the diversity of sizes, shapes or densities among different fractions of mitochondrial nucleoid-membrane complexes may be caused by the different amounts of membrane components association with each fraction, respectively.
The mtDNA contents varied among mitochon-drial nucleoid-membrane complexes with different densities from a sucrose gradient frac-tionation
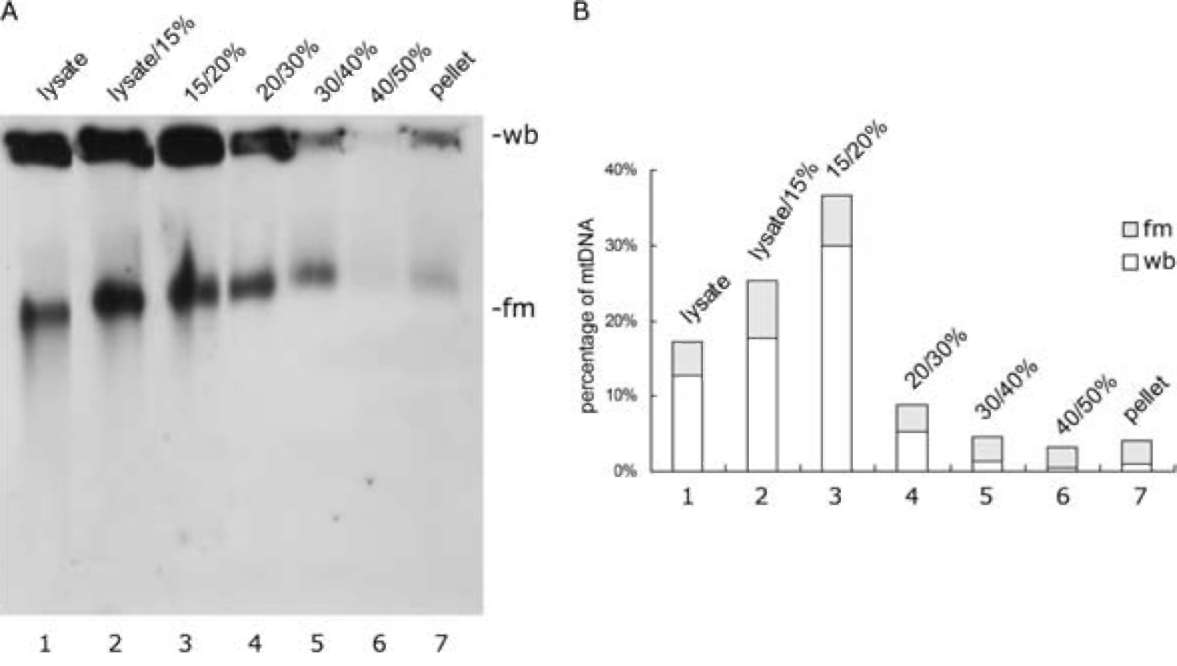
Southern blot analysis of mitochondrial nucleoid-mem-brane complexes revealed that 36.6%, 25%, 17%, 8.9% and 4.55% of the original mtDNA was present, respectively, in the 15/20% sucrose boundary, lysate (7.5%)/15%
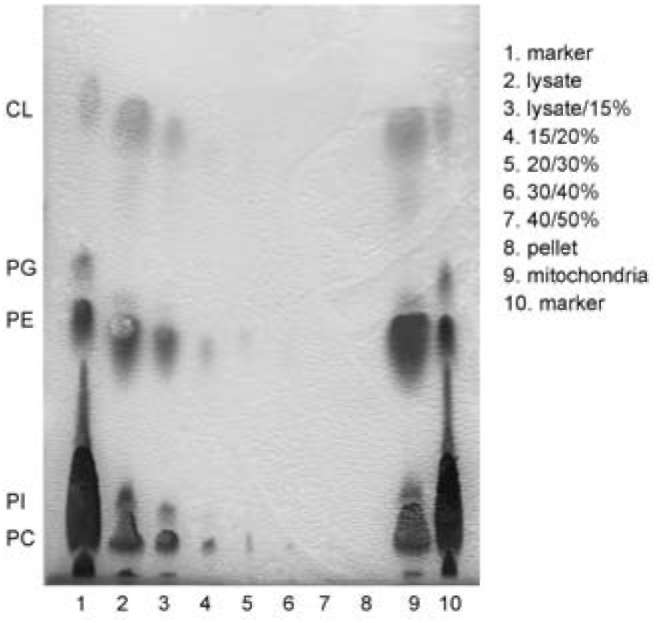
Figure 1. Phospholipid compositions of mitochondrial nucleoid-membrane complexes in different fractions after sucrose gradient centrifugation. Equivalent portions of each of the seven mitochondrial nucleoid-membrane complex fractions indicated on the right of the figure were analyzed by thin layer chroma-tography. A phospholipid sample extracted from the purified mitochondria containing 0.5 mg proteins was loaded in line 9 as the control. The phospholipid markers in lanes 1 and 10 are car-diolipin (CL), phosphoglycerol (PG), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidylcholine (PC).
sucrose boundary, the lysate layer (7.5% sucrose), 20-30% sucrose boundary and 30-40% boundary (Figure 2, Table 1). In addition, the ratios between the well-bound (wb) form and the fast-moving (fm) form of mtDNA also varied from one sucrose gradient fraction to another (Figure 2B). The highest ratio between wb form DNA to fm form DNA was found in the fraction of 15/20% sucrose boundary (Figure 2B).
In vitro mtDNA replication by the different mito-chondrial nucleoid-membrane complexes
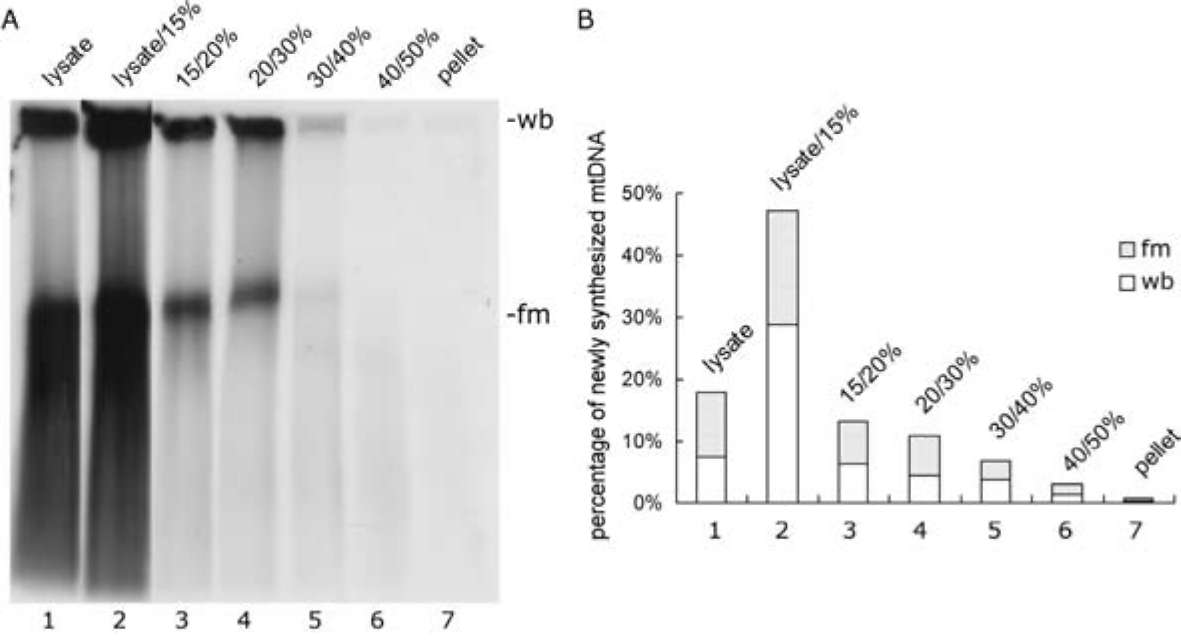
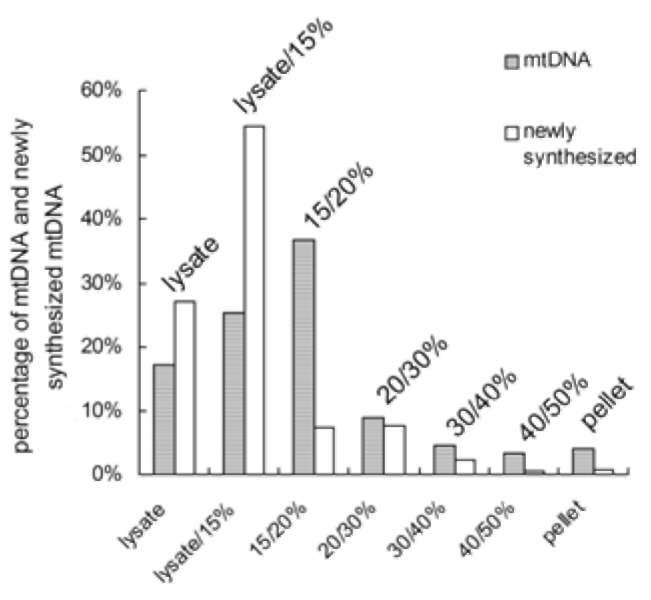
In vitro mitochondrial nucleoid-membrane complex DNA replication was performed as described in Materials and Methods. After PFGE analysis, the complexes harvested from the lysate/15% sucrose boundary showed the most mtDNA synthesis, even though its mtDNA content was less than that in the 15/20% fraction (Figure 3 and Table 1). In addition, the wb to fm ratio of the newly synthesized mtDNA was much lower than that of the original template mtDNA (compare Figure 3, Panel B to Figure 2, Panel B). Self-sufficient mtDNA replication was found in all seven fractions, and even in the pellet, which was also able to accomplish mtDNA synthesis/replication. Newly synthesized mtDNA could barely be detected in the 40/50% boundary fraction. This result indicates that the mtDNA-protein-membrane complexes in these seven fractions carried the essential factors for DNA synthesis.