JIANG et al. — Allopolyploidization induced the activation of Tyl-copia retrotransposons in Cucumis allotetraploid 147
Genomic PCR and RT-PCR analysis
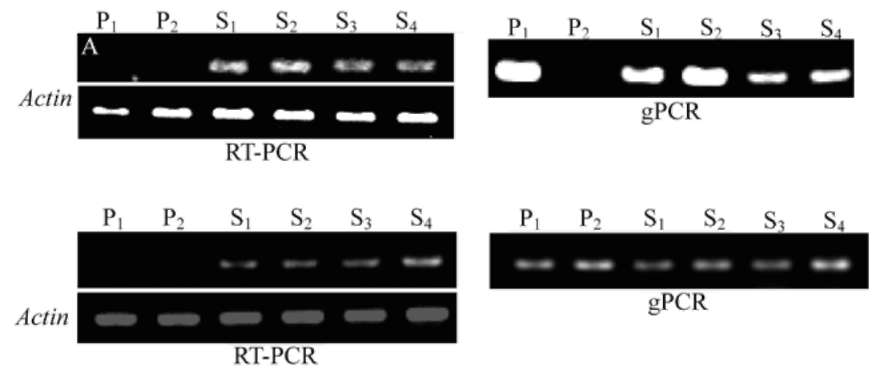
To verify the expression of the cloned reverse tran-scriptase sequences in the first four generations of allotetraploid, genomic PCR (gPCR) and RT-PCR using specific primers were carried out respectively. We tried to design specific primers based on all the 13 intact clones. RT3 and RT10 belonging to different groups of retrotransposons in C. hytrix were chosen as target fragments for gPCR and RT-PCR. The PCR condition was followed as: 3 min at 94°C; 30 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C, fol-lowed by 35 cycles. Actin gene was used as positive con-trol in the RT-PCR reactions.
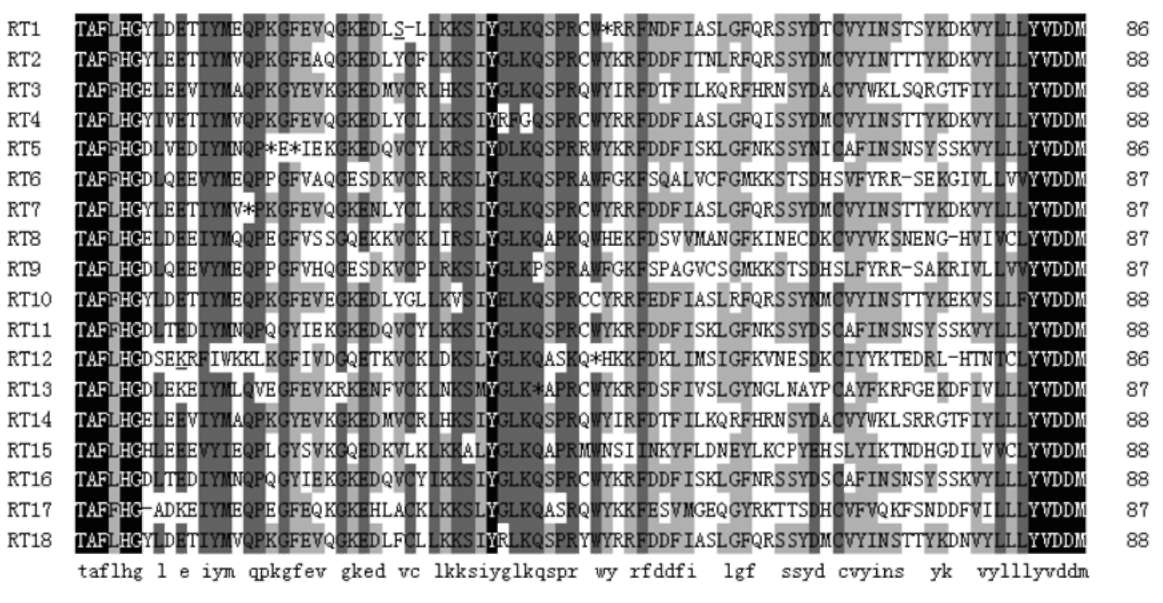
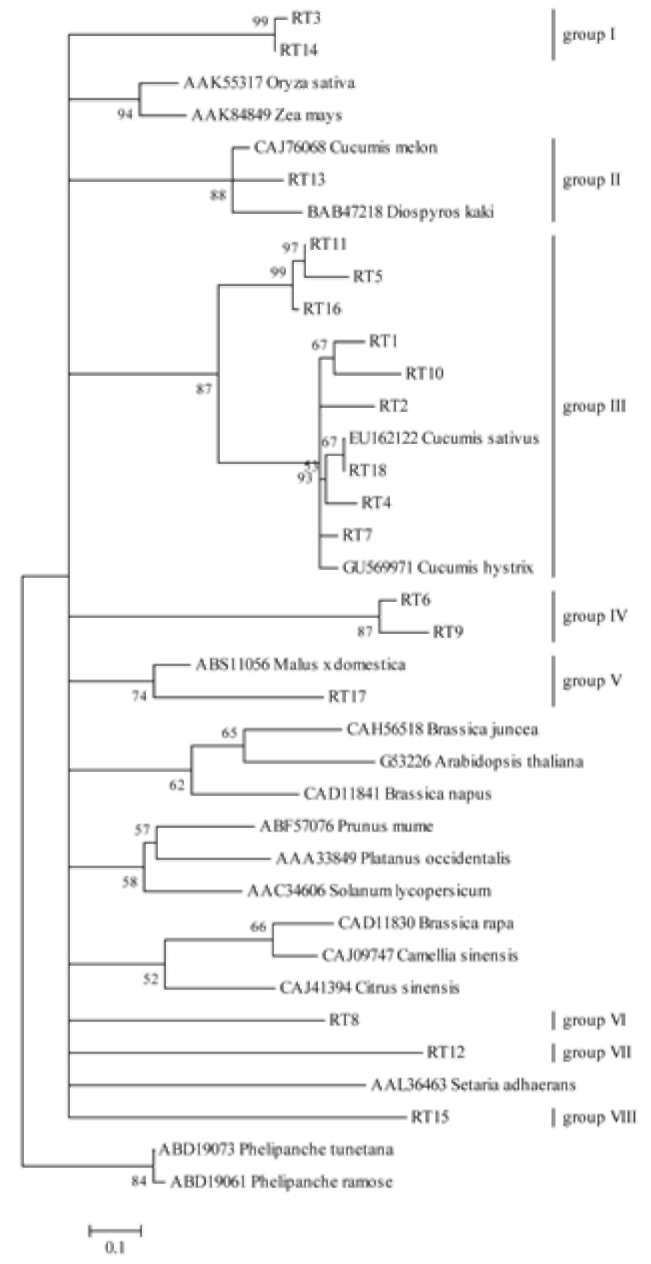
other, we confirmed that these sequences were highly heterogeneous. The 18 sequences were conceptually translated and where necessary edited for frameshift, based on published residue landmarks of plant retrotransposons' RT domains. The amino acid alignments had properly translated primer sequences at both ends. Among these 18 sequences, 5 (27.8%) contained premature stop codons and/or frameshift in their coding regions (RT1, RT5, RT7, RT12, and RT13). The remaining 13 sequences (72.2%) were not affected by either stop codons or frameshift. The homology ranged from 49.4% (RT4 and RT9) to 98.1% (RT3 and RT14) when amino acid sequences of these RTs
RESULTS
Identification of active retrotransposons in Cucumis
Transcriptional active retrotransposons were tested by RT-PCR from allotetraploid and its two diploid parents, C. hystrix and C. sativus. Only the allotetraploid yielded a specific product with expected size (260 bp) (Figure 1). The amplified fragment was recovered and cloned into a pGEM-T vector, and 20 clones were obtained. Sequence analysis revealed that 18 clones contained the RT domains of the retrotransposons (named as RT1 to RT18), while the remaining 2 didn't. These sequences have already been submitted to GenBank with the accession numbers of HMO036494-HMO036511.
RT sequence alignments
Comparing the 18 newly-isolated sequences with each
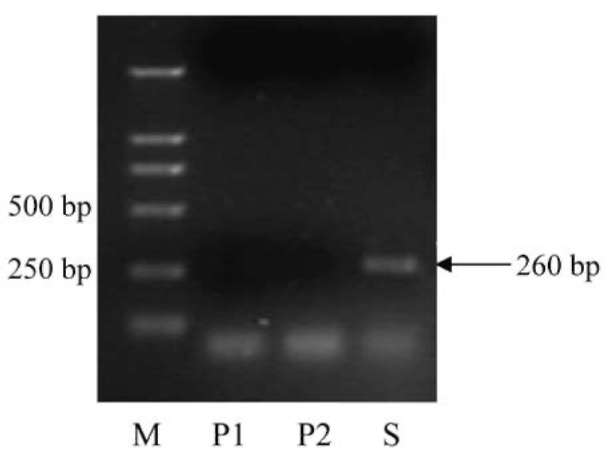
Figure 1. RT-PCR amplification of reverse transcriptase (RT) domains of Ty 1 -copia retrotransposons from genomes of allotetraploid and its two diploid parents. M: 2000 bp Marker; P1: Cucumis hystrix; P2: C. sativus; S: C. hytivus.
Table 1. Homology matrix of the 18 reverse transcriptase (RT) sequences of active retrotransposons amplified from allotetraploid C. hytivus.
|
|
RT1
|
RT2
|
RT3
|
RT4
|
RT5
|
RT6
|
RT7
|
RT8
|
RT9
|
RT10
|
RT11
|
RT12
|
RT13
|
RT14
|
RT15
|
RT16
|
RT17
|
||
|
RT2
|
82.2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
RT3
|
60.4
|
63.5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
RT4
|
86.4
|
83.8
|
61.7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
RT5
|
67.8
|
68.8
|
60.5
|
66.5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
RT6
|
53.3
|
57.0
|
53.6
|
53.2
|
54.8
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
RT7
|
86.0
|
89.5
|
63.2
|
85.7
|
65.0
|
54.4
|
|
|
|
|
|
|
|
|
|
|
|
||
|
RT8
|
57.3
|
57.3
|
57.3
|
58.4
|
58.0
|
52.9
|
57.6
|
|
|
|
|
|
|
|
|
|
|
||
|
RT9
|
51.7
|
53.6
|
53.2
|
49.4
|
51.7
|
90.1
|
53.2
|
51.3
|
|
|
|
|
|
|
|
|
|
||
|
RT10
|
86.4
|
81.6
|
59.8
|
85.3
|
67.7
|
52.5
|
83.1
|
56.9
|
49.0
|
|
|
|
|
|
|
|
|
||
|
RT11
|
67.4
|
68.4
|
63.9
|
65.4
|
91.0
|
55.5
|
65.0
|
56.1
|
54.4
|
66.2
|
|
|
|
|
|
|
|
||
|
RT12
|
53.6
|
55.9
|
56.3
|
53.6
|
56.7
|
51.5
|
55.1
|
68.8
|
51.5
|
53.2
|
58.2
|
|
|
|
|
|
|
||
|
RT13
|
59.1
|
59.4
|
56.8
|
57.1
|
57.1
|
52.5
|
59.0
|
56.1
|
48.7
|
54.1
|
57.9
|
56.3
|
|
|
|
|
|
||
|
RT14
|
61.0
|
63.5
|
98.1
|
62.4
|
60.9
|
53.6
|
63.9
|
57.6
|
53.2
|
60.5
|
62.8
|
55.9
|
58.6
|
|
|
|
|
||
|
RT15
|
57.6
|
54.9
|
55.6
|
53.4
|
53.0
|
52.9
|
56.0
|
57.6
|
51.7
|
53.4
|
54.1
|
52.1
|
51.9
|
56.4
|
|
|
|
||
|
RT16
|
69.7
|
69.9
|
63.2
|
66.9
|
90.6
|
55.1
|
66.5
|
56.9
|
52.9
|
66.9
|
94.4
|
59.7
|
59.8
|
63.2
|
54.5
|
|
|
||
|
RT17
|
58.6
|
60.4
|
57.7
|
57.7
|
60.4
|
57.3
|
59.2
|
59.8
|
55.7
|
57.4
|
59.2
|
55.0
|
61.5
|
57.7
|
58.1
|
58.9
|
|
||
|
RT18
|
89.4
|
83.8
|
62.4
|
91.7
|
65.0
|
52.9
|
86.1
|
59.5
|
51.3
|
86.1
|
66.9
|
54.8
|
56.8
|
62.0
|
55.6
|
66.2
|
59.2
|
||