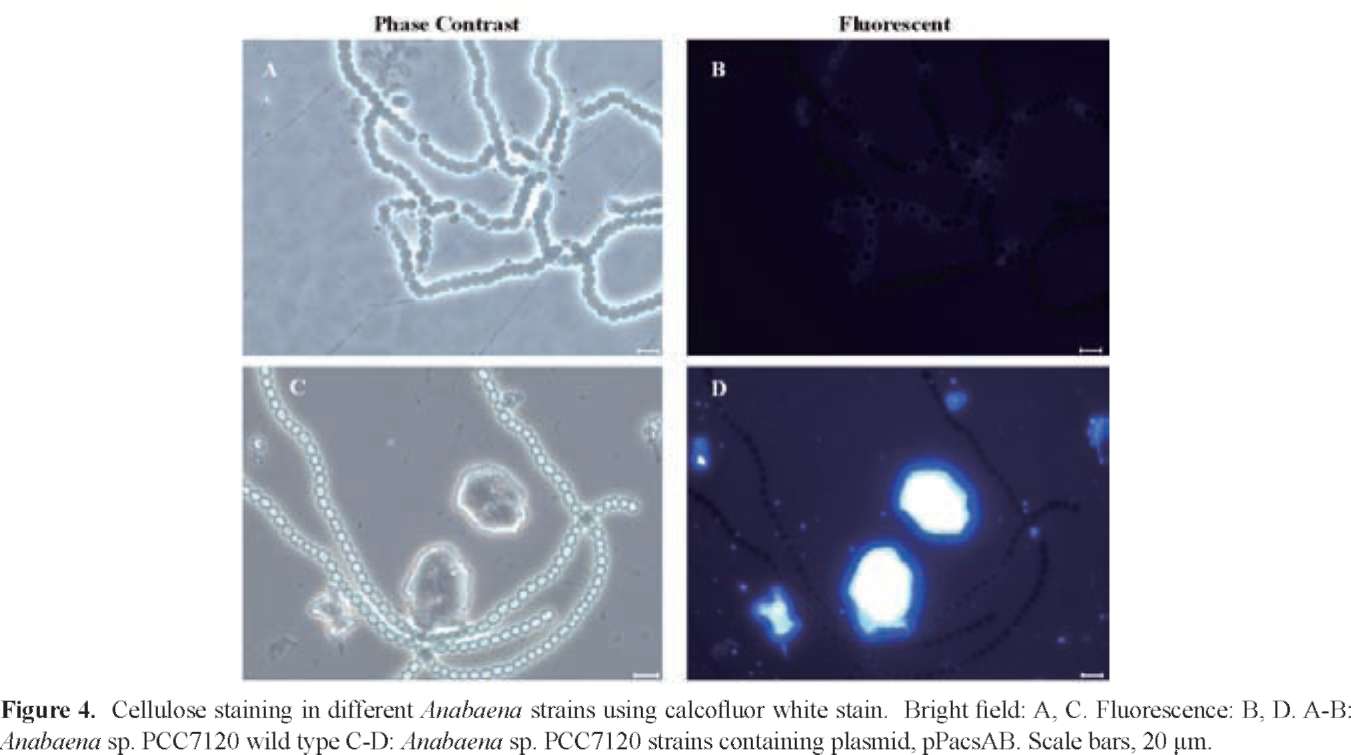
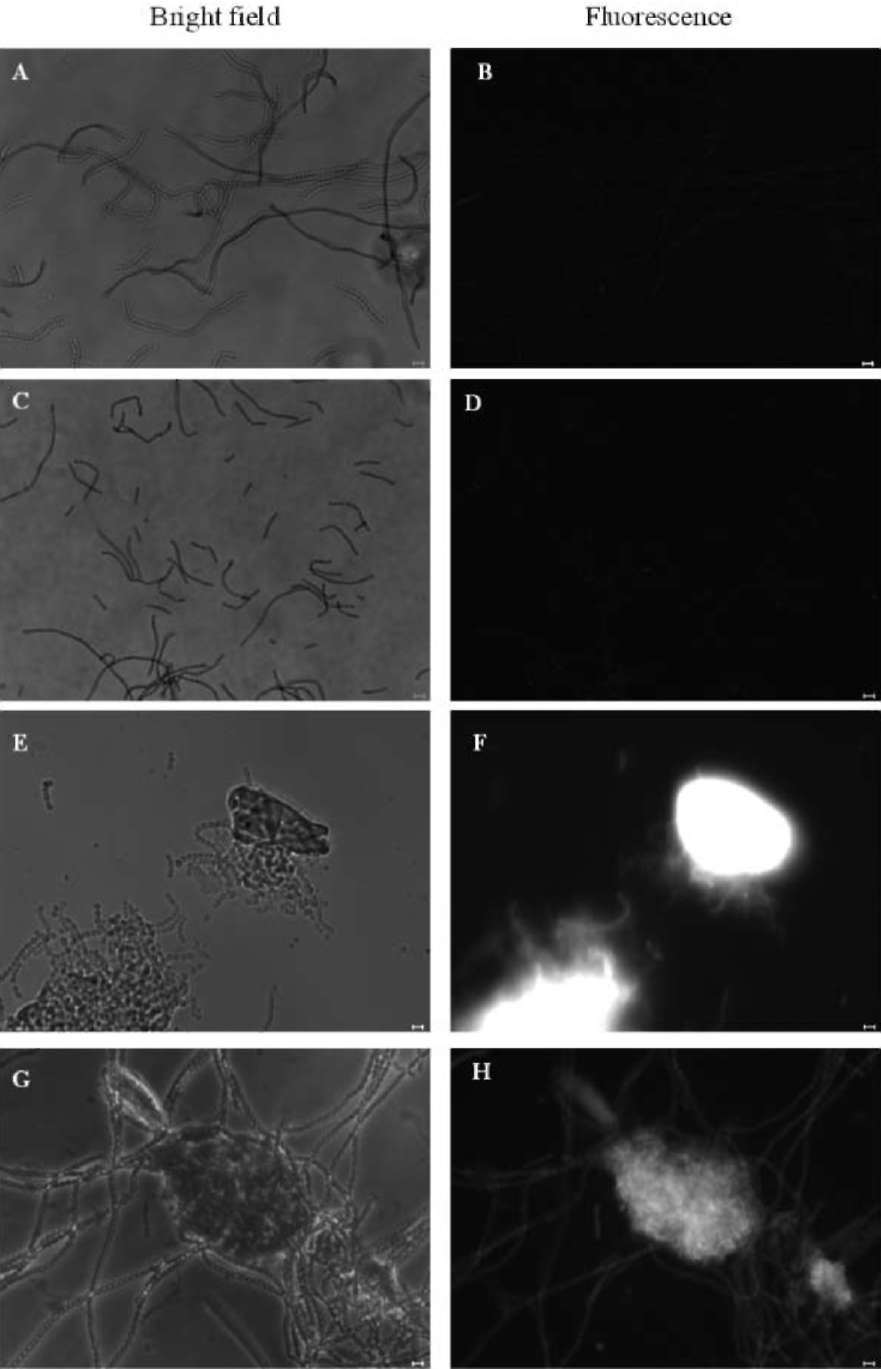
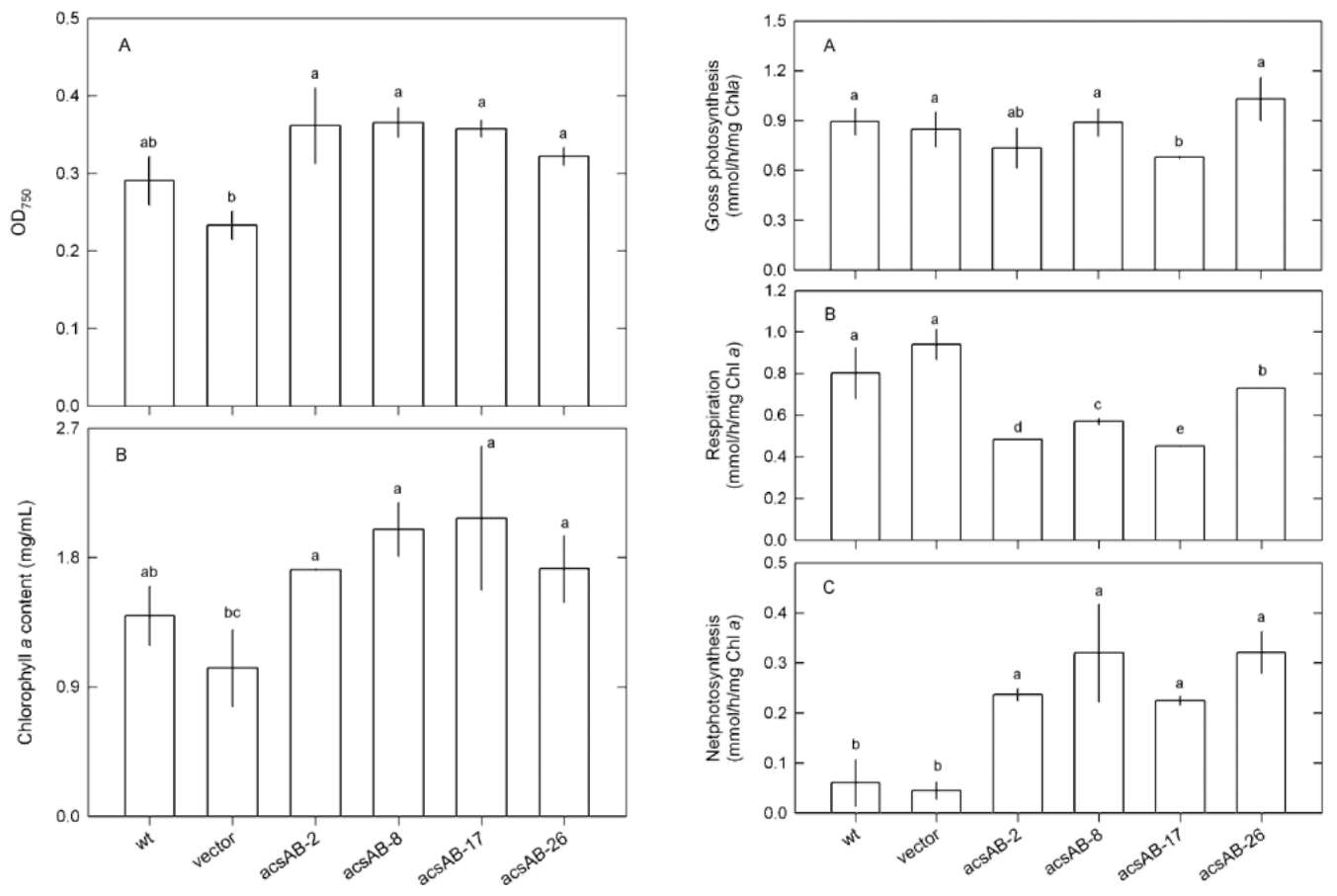
SU et al. ― Enhanced cellulose production in Anabaena 267
|
Table 1. Bacterial strains and plasmids used.
|
||||
|
Strain or plasmid
|
Relevant characteristic (s)
|
Source or reference
|
||
|
Strains of Anabaena sp.
|
|
|
||
|
PCC 7120
|
Wild type
|
PCCa
|
||
|
Strain acsAB-2
|
PCC 7120 carrying pPacsAB
|
This study
|
||
|
Strain acsAB-8
|
PCC 7120 carrying pPacsAB
|
This study
|
||
|
Strain acsAB-17
|
PCC 7120 carrying pPacsAB
|
This study
|
||
|
Strain acsAB-26
|
PCC 7120 carrying pPacsAB
|
This study
|
||
|
Strain pRL489-Nmr
|
PCC 7120 carrying pRL489-Nmr
|
This study
|
||
|
Strains of E. coli
|
|
|
||
|
DH5a
|
Recipient in transformations
|
|
||
|
Strains of A. xylinum
|
|
|
||
|
A. xylinum ATCC23769
|
Source of acsAB gene
|
ATCCb
|
||
|
Plasmids
|
|
|
||
|
pAcsAB
|
yT&A containing 4.6 kb acsAB gene
|
This study
|
||
|
pRL489-Nmr
|
pRL489 removing luxAB gene
|
This study
|
||
|
pPacsAB
|
pRL489-Nmr containing acsAB gene
|
This study
|
||
|
pRL489
|
Kmr Nmr; pDU1-based shuttle vector; carries PpsbA-luxAB
|
Elhai, J.
|
||
|
pRL443
|
Apr Tcr; conjugative plasmid
|
Elhai, J. & C. P. Wolk
|
||
|
pRL528
|
Cmr; helper plasmid; carries mob
|
Elhai, J. & C. P. Wolk
|
||
|
yT&A
|
Apr; cloning vector
|
Yeastern Biotech Co.
|
||
aPCC, The Pasteur Culture Collection of Cyanobacteria, Institut Pasteur, France.
bATCC, American Type Culture Collection, Rockville, MD, USA.
Triparental conjugative transfer
Conjugation between E. coli and Anabaena was performed as described (Elhai and Wolk, 1988). Briefly, two strains of E. coli HB101: one containing conjugation plasmid, pRL443, and the other containing helper plas-mid, pRL528, along with either shuttle vector pPacsAB, or pRL489-Nmr (control), were mixed with Anabaena sp. PCC 7120 and spotted onto filter papers laid on BG-11 agar plates without antibiotic. After incubation for 48 h, the filters were transferred to plates supplemented with 25 [ig/mL neomycin (Nm) and incubated until Nmr exconju-gant colonies appeared. The cyanobacterial clones, which appeared on the filters after about two weeks, were picked and restreaked on BG-11 plates containing 25 [ig/mL neo-mycin (Nm). Putative exconjugates were transferred to BG-11 liquid medium containing Nm (15 [g/mL) for more rapid cultivation and further analysis.
Shuttle vector construction
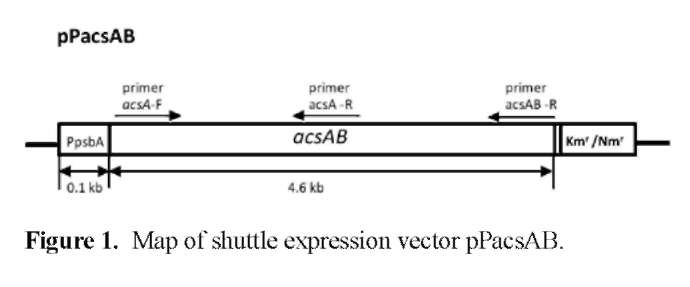
A 2 kb BamHI fragment containing the luxAB sequence was removed from pRL489 (Elhai, 1993) and a 4.6 kb BamHI fragment from pAcsAB, containing the acsAB genes from A. xylinus strain ATCC 23769, was ligated into the BamHI site of pRL489 to create a shuttle plasmid pPacsAB, where the acsAB sequence was oriented in the same direction as the psbA promoter (Figure 1).
The 2 kb BamHI fragment containing the luxAB sequence was removed from pRL489 and the remaining fragment was self-ligated to generate plasmid pRL489-Nmr. Plasmid pRL489-Nmr was used as a negative control in detecting acsAB expression in acsAB transgenic cells. The resulting plasmids, pRL489-Nmr and pPacsAB, were confirmed by restriction digestion, and used for conjuga-tive transfers.
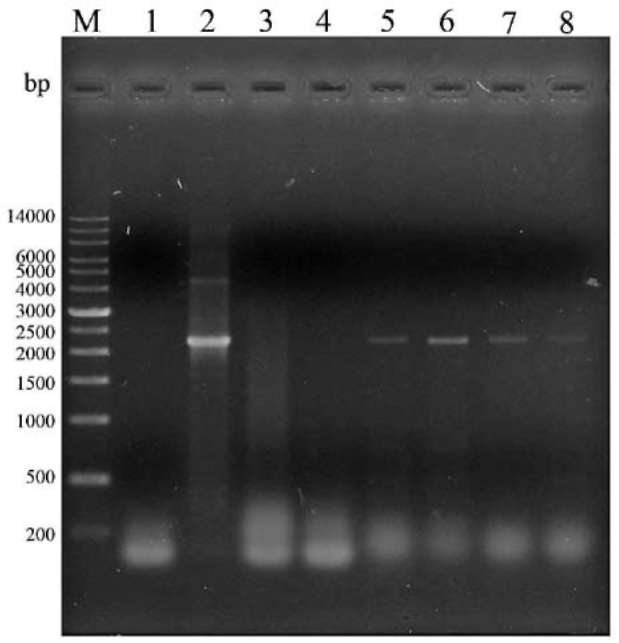
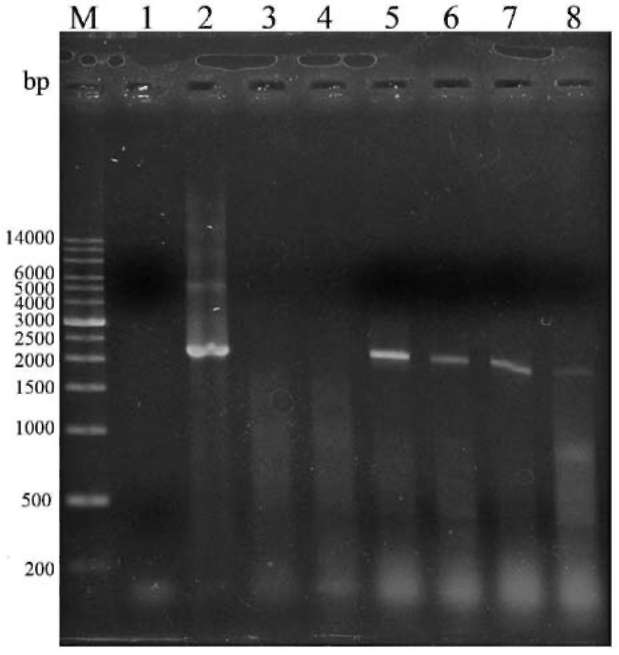
PCR screens for transgenic cells
Cultures of Anabaena sp. PCC7120 harboring either plasmid pRL489-Nmr or pPacsAB were used in PCR screens to detect acsAB. One mL liquid culture of each strain was pelleted by centrifugation, resuspended in 200 fiL of TE, pH 8.0 supplemented with 1% Triton X-100, and lysed by heating for 2 min at 95°C (Hagen and Meeks, 1999). The lysate was then extracted twice with an equal