286
Botanical Studies, Vol. 52, 2011
Culture conditions and data analysis
Uniform culture conditions were applied in all experiments. The pH of the media was adjusted to 5.7 before autoclaving. The media was autoclaved for 15 min at 1.05 kg/cm2 pressure at 121°C. Cultures were incubated at 25±1°C in the dark. All experiments were repeated three times with 20 replicates each time. All data were analyzed using standard applied method.
Callus induction from different explants
Different plant parts (leaf, stem, petiole and root) were used as explants for the callus induction. The explants were cut into small pieces and inoculated into different media viz., MS (Murashige and Skoog, 1962), N6 (Chu et al., 1975), woody plant (Lloyd and McCown, 1980) and B5 (Gamborg et al., 1966). These media were further supplemented with 3% sucrose and different concentra-tions (0.5 to 2.0 mg/L) of plant growth regulators: auxins (2,4 diphenoxy acetic acid {2,4-D}, indole acetic acid {IAA}, naphthalene acetic acid {NAA}) and cytokinins (6-Benzyladenine {BA}, kinetin, thiodiazuran {TDZ} and zeatin). The callus formation was observed from the cut surface 10-15 days after culture.
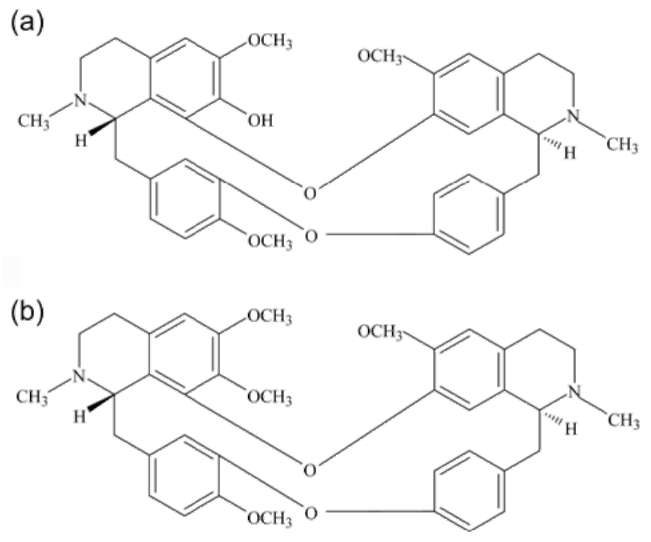
Figure 1. Chemical structure of (a) fangochinoline (b) tetrandrine.
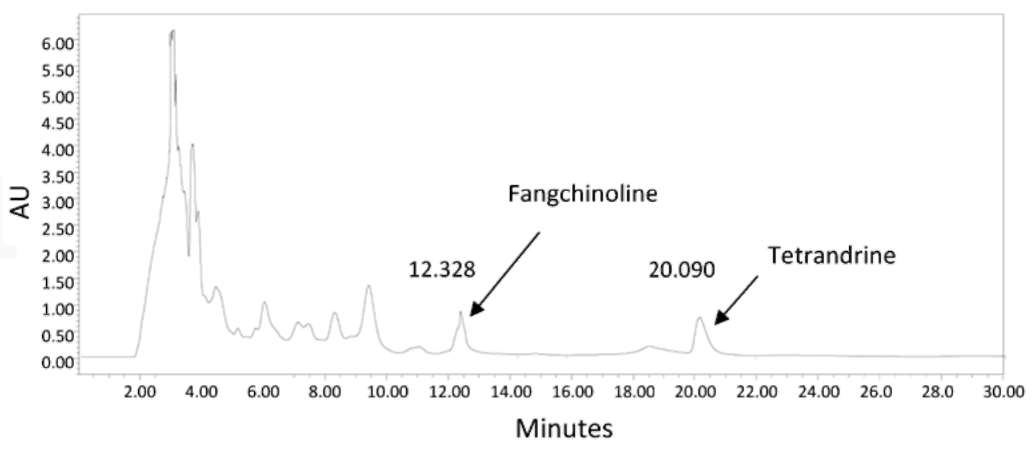
cological activities (Ou, 1992) and methods such as high-performance liquid chromatography (HPLC) (Huang and Hong, 1998), thin-layer chromatography (TLC) (Lin et al., 1993), HPTLC (Blatter and Reich, 2004), capillary electrophoresis (CE) (Yang et al., 1998) non-aqueous CE (Li et al., 2004), flow injection-micellar electrokinetic capillary chromatography (FI-MEKC, Liu et al., 2005) and mass spectrometry (Koh et al., 2006) have been are used to detect the presence of Fan and Tet.
One of the reasons for research using in vitro cultures of various plant cells, tissues or organs is their ability to synthesize metabolites in higher concentration under the influence of some additives as compared to whole plants (Panda et al., 1991; Fennell et al., 2003). This becomes an alternative for obtaining products that are difficult to obtain by conventional methods or are not economically viable. Furthermore, plant metabolites can vary due to climatic and seasonal conditions. Thus, it becomes imperative to grow the plants in a controlled environment. The aim of the present work was to establish the mass production of callus and analysis of pharmaceutically important alkaloids such as Fan and Tet under the influence of different additives.
Callus growth determination
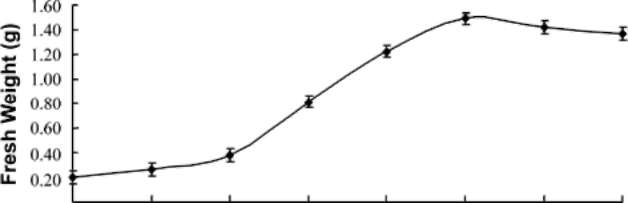
Leaf callus stocks were used to determine the growth curves. Pieces of friable callus (~0.2 gm) were inoculated in the MS medium supplemented with 1 mg/L of BA, 0.5 mg/L TDZ and 3% sucrose in the dark at 28±1°C. Weight of growing callus was recorded at an interval of four days for 45 days and a growth curve was plotted based on the fresh weight of callus over the respective time period.
Enhanced callus formation
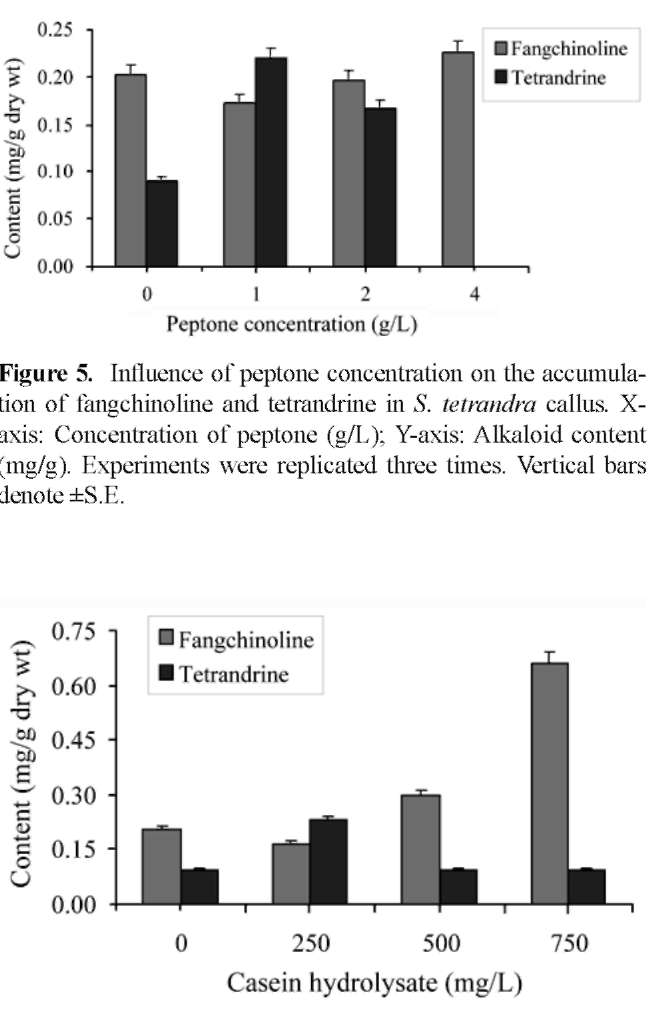
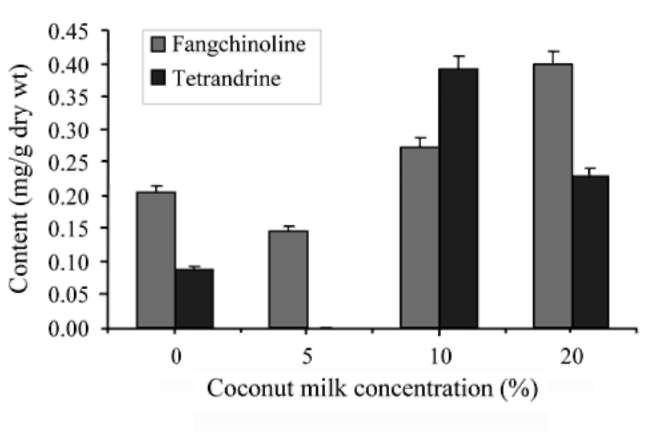
The growth of callus was further enhanced by supplementing the previous media with 250-750 mg/L casein hydrolasate (Fluka analytical. USA), 5-20% coconut milk (Vegetable market, Taichung) and 1.0-4.0 g/L peptone
materials and methods
Plant material and sterilization
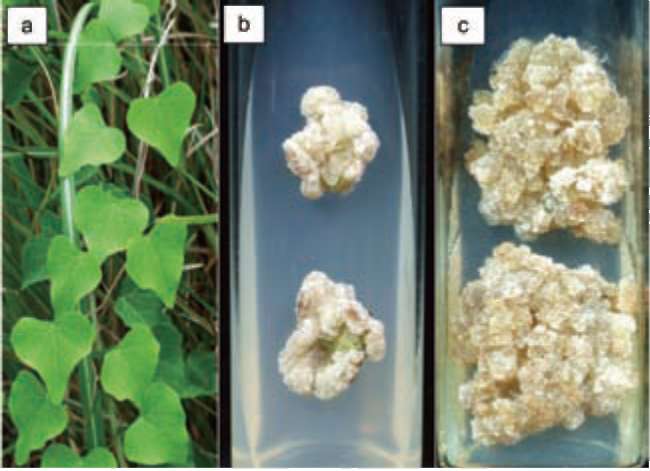
Plants of Stephania tetrandra S Moore (Han fang ji, Figure 2) were collected from Da-Du Mountain in Taic-hung County (Taiwan). The explants were washed with running tap water followed by surface sterilization with 70% v/v ethanol for 30's'. Explants were then washed three times with sterile distilled water to remove traces of ethanol and sterilized with 0.5% (w/v) sodium hypochlo-rite for 7 min in an ultrasonic oscillator (15 amplitude). Explants were rinsed five times with sterilized water to remove traces of hypochlorite.
Figure 2. Callus induction and proliferation in Stephania tet-randra. (a): Plant in natural habitat; (b): Callus induction from leaf; (c): Proliferated callus.