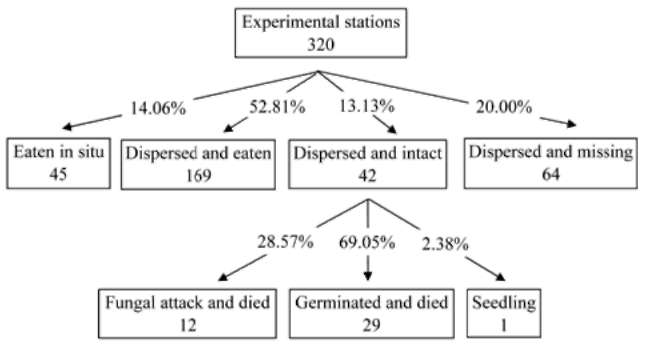
CHOU et al.— Seed fate of Castanopsis indica 323
dispersed and buried in the fern root system); (4) in the soil layer (tagged seeds were dispersed and buried in the soil); (5) under a fallen tree or branch (tagged seeds were dispersed on the ground and covered by fallen tree or branch); and (6) in a fallen tree hole (tagged seeds were dispersed in the tree hole of a fallen tree) (modified from Li and Zhang, 2003). Furthermore, we measured the distance (straight line) of the tagged seeds or their fragments from the original release stations to the places that they were found. We then reburied the seeds in the cache site, attempting to keep disturbance to a minimum. To determine whether the dispersed seeds were randomly distributed among microhabitat characteristics, seed counts per microhabitat were made and compared to the total cover for that microhabitat using a Chi-square test.
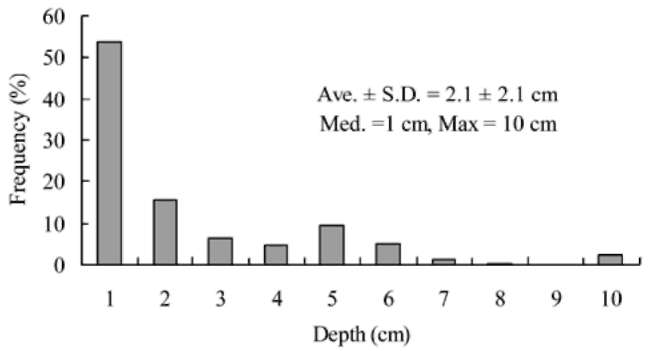
Figure 2. Frequency distribution of the burying depths of 211 hoarded seeds by spinous country-rats (Niviventer coxingi).
the experiment stations from which they were initially released, and the maximum dispersal distance was 21.6 m. A greater proportion (45%) of dispersed seeds or seed fragments was distributed within 5 m (Figure 1). For all found tagged seeds, the median dispersed distance was 6.2 士 4.9 m (士 S.D., mean = 7 m, maximum = 21.6 m, n = 211).
RESULTS
Seed predators and dispersers census
On the iron tower, the four automatic cameras took 144 photographs, including five pictures of Formosan giant flying squirrels (Petaurista philippensis), which are olfactorily-oriented nocturnal animals, and three pictures of red-bellied tree squirrels (Callosciurus erythraeus). The video camera captured 72 h of video images, including consuming films of Formosan macaques (Macaca cy-clopis) and red-bellied tree squirrels, which are visually-oriented diurnal animals. Automatic cameras took 140 pictures of rodents at the four seed experimental stations, but the spinous country- and Formosan white-bellied rats' (Niviventer culturatus) close resemblance prevented their true identification. Because a spinous country-rat (Niviventer coxingi) was trapped during the seed dispersal experiment, we judged the photographed rodents to be the same species. Spinous country-rats played major roles as seed predators and scatter-hoarding seed dispersers in the study site.
Microhabitat characteristics
The buried depth distribution of 211 tagged seeds was right-skewed and leptokurtic (Figure 2). A large proportion (54%) of the found seeds or their fragments was found within 1 cm depth. The median depth of buried seeds was 1 士 2.1 cm (士 S.D., mean = 2.1 cm, maximum = 10 cm, n = 211). The frequency distribution of the microhabitat characteristics where the tagged seeds or their fragments were dispersed by the spinous country-rat are shown in Figure 3. The dispersed seeds were not randomly distributed among the different microhabitat characteristics (X2 =146.2, P<0.001, df=5). A large proportion (60.2%, 127/211) of the buried seeds or seed fragments was distributed in the litter layer. Secondly, 15.6% (33/211) and 13.3% (28/211) of the buried seeds or seed fragments were distributed on bare ground and in the soil layer, re-spectively. Furthermore, 7.1% (15/211) and 2.8% (6/211) of the buried seeds or their fragments were distributed in the root system or under a fallen tree or branch layer, re-
Dispersal distance
The dispersal distance of tagged seeds was quite short. Most of the tagged seeds were found within 20 m of
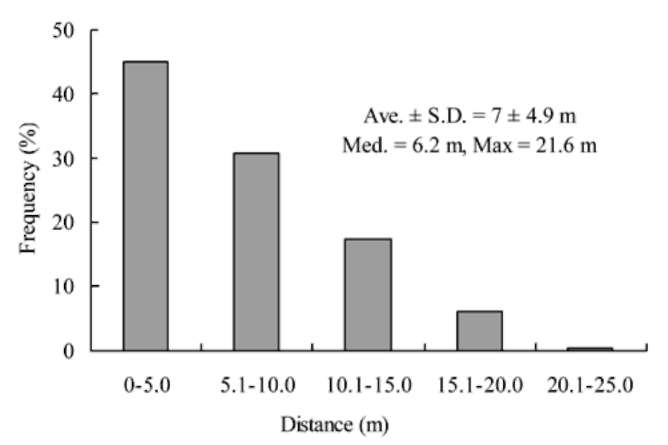
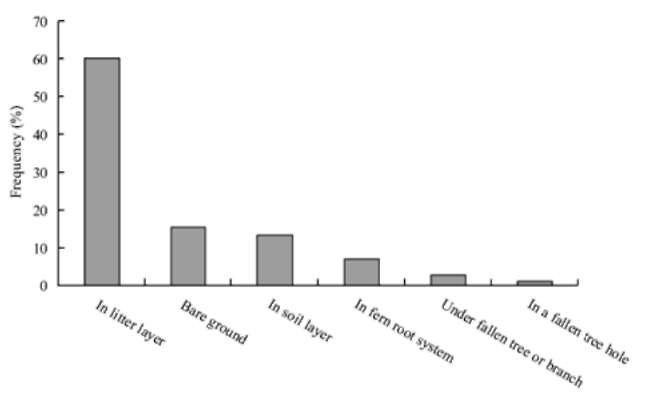
Figure 3. Frequency distribution of the microhabitat characteristics made by spinous country-rats (Niviventer coxingi) for 211 hoarded seeds.
Figure 1. Distance distribution of 211 seeds dispersed by spinous country-rats (Niviventer coxingi).