328
Botanical Studies, Vol. 52, 2011
gated, because dispersal distances for many tree species are short, thus, seeds are mostly deposited near their parents (Clark et al., 1998; Dalling et al., 2002; Svenning and Wright, 2005). As a consequence of such dispersal limitation, many seeds may fail to reach microhabitats suitable for germination and greatly impact subsequent seedling dynamics. Furthermore, seed distributions may reflect topographic features of forests. It has been demonstrated that the process of seed dispersal can be greatly altered by topography (Schurr et al., 2008). Topography may also influ國 ence seed distributions indirectly via parent distributions. Recent studies in several, large, forest dynamics plots have found that the majority of tree species in tropical forests are aggregated at the stand level (Condit et al., 2000; Sei-dler and Plotkin, 2006). Such aggregation of tree species may further reinforce seed aggregations at the stand level.
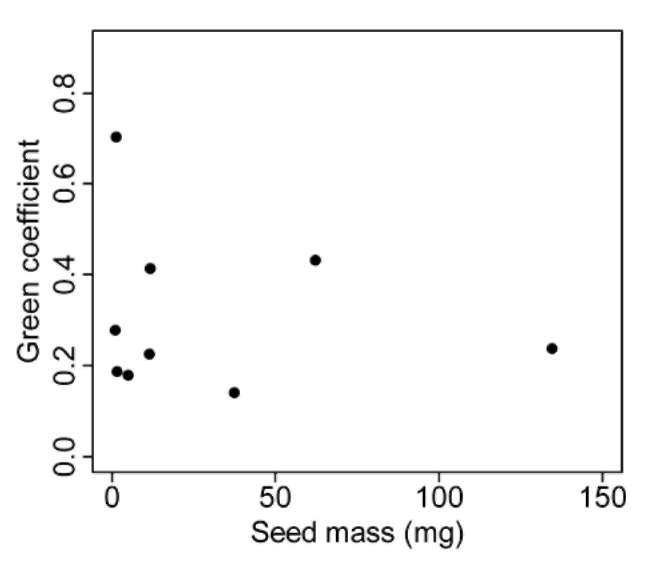
To what extent can seed distribution be predicted by species attributes associated with seed dispersal? The process of seed dispersal is closely related to seed traits, such as dispersal mode and seed mass (Fenner and Thompson,2005). Muller-Landau et al. (2008) found that dispersal mode explained some of the interspecific variation in seed distributions. Many studies have shown that different dis-persal agents result in different patterns of seed distribu-tion (Clark et al., 1999; Clark et al., 2005; Muller-Landau et al., 2008). In addition, seed mass explained interspecific variation in dispersal distances and seed production in many tree species in a tropical forest in Panama (Muller-
Landau et al., 2008).
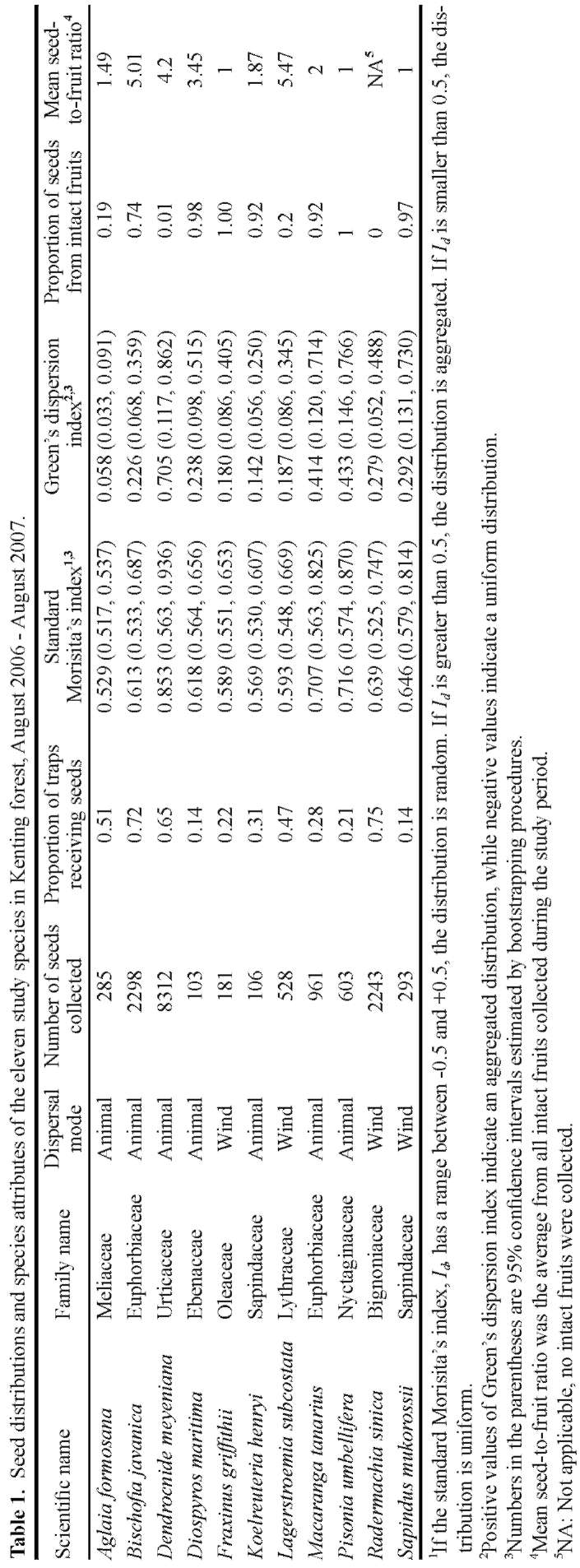
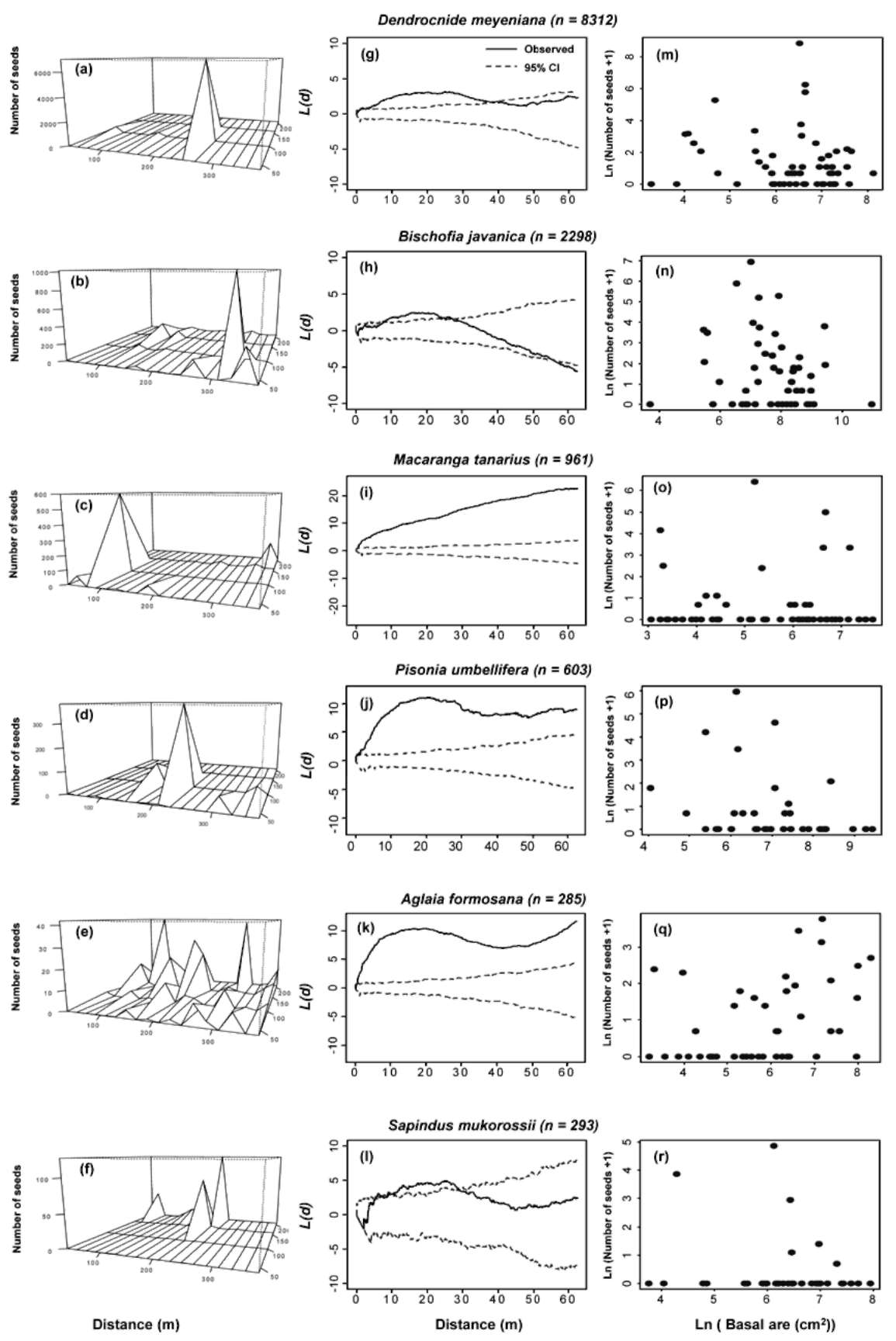
In this study, we documented, at the stand level, the seed distributions of eleven tree species in a tropical forest in Taiwan. We investigated how seed distributions are related to parent tree distributions, topography and seed traits. We found that seeds tend to be aggregated at the stand level. Seed aggregations may arise from short dispersal distances from parent trees and reflect the local topography. Topography may influence seed distributions directly, or indirectly via the distributions of parent trees. Finally, seed mass and dispersal mode may account for most of the interspecific variability in seed distributions.
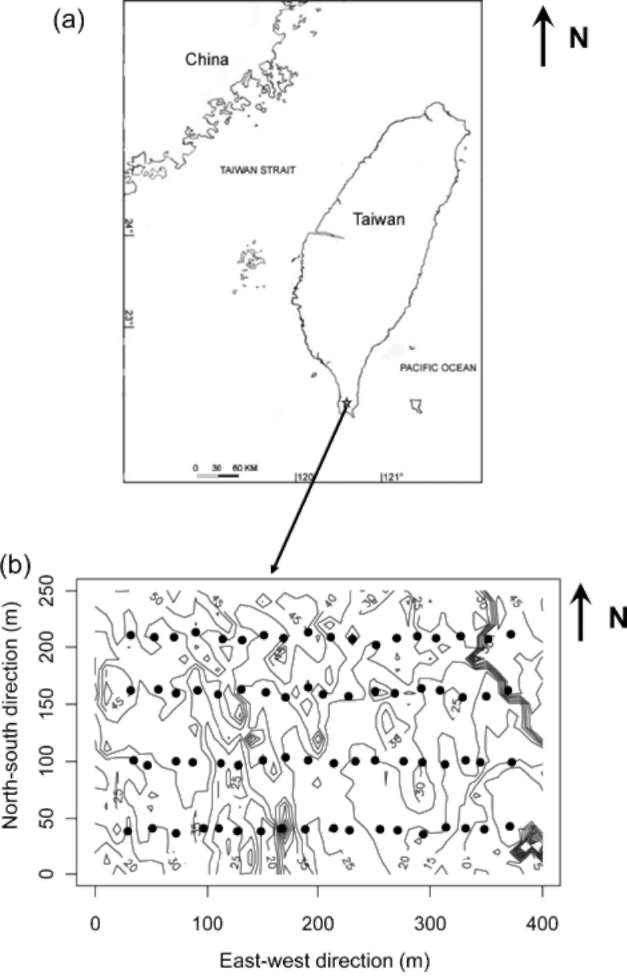
Figure 1. Map of the study site in Kenting, Taiwan. (a) The location of Kenting is marked by an asterisk. (b) Locations of seed traps within the Kenting forest dynamics plot. The black dots represent seed traps with 10 m contour lines in the background.
is especially abundant, accounting for 50.3% of the individuals within the plot (Yu, 1998; Wang et al., 2004). Other common species include swamp gelonium (Melanolepis multiglandulosa), autumn maple tree (Bischofiajavanica), Taiwan nato tree (Palaquium formosanum), sappan wood (Aglaia formosana), and pouteria (Pouteria obovata). The mean canopy height is about 10 m.
MATERIALS AND METHODS
Study area
This study was carried out in Kenting Elevated Coral Reef Natural Reserve on the Hengchun Peninsula (21°58' N, 120°48' E), Taiwan (Figure 1). This site is underlain by Miocene rocks and capped with an elevated coral reef (Wang et al., 2004). There is a distinct monsoon season from October to March (Wang et al., 2004). The average mean temperature is 25.1°C and average annual precipitation is 1963.8 mm (1977-2006). A 10 ha (400 m x 250 m) forest dynamics plot (FDP) was established in 1997 (Wang et al., 2004). The plot is dominated by woody species in the Ebenaceae and Euphorbiaceae. One hundred and ten woody species were found within the forest dynamics plot (Wang et al., 2004) Coast persimmon, Diospyros maritima,
Seed rain collection
We established four east-west transects within the 10 ha permanent plot (Figure 1). Each transect was 340 m long, starting at a point 30 m from the western edge of the plot and ending 30 m from the eastern edge. Areas within 30 m from the plot edges were excluded to avoid edge effects. The transects were 50 or 60 m apart in the south-north direction. The distances between transects were determined by local topographic features. Areas with extreme topographic differences were avoided. A total of 72 seed traps were placed, at 20 m intervals, along the four transects (Figure 1). Locations with heavy understory vegetation or bare rock were avoided because it was difficult to set