HSIEH et al. ― Classifier modeling and numerical taxonomy of Actinidia in Taiwan
341
RESULTS
Classification of Actinidia accessions in Taiwan
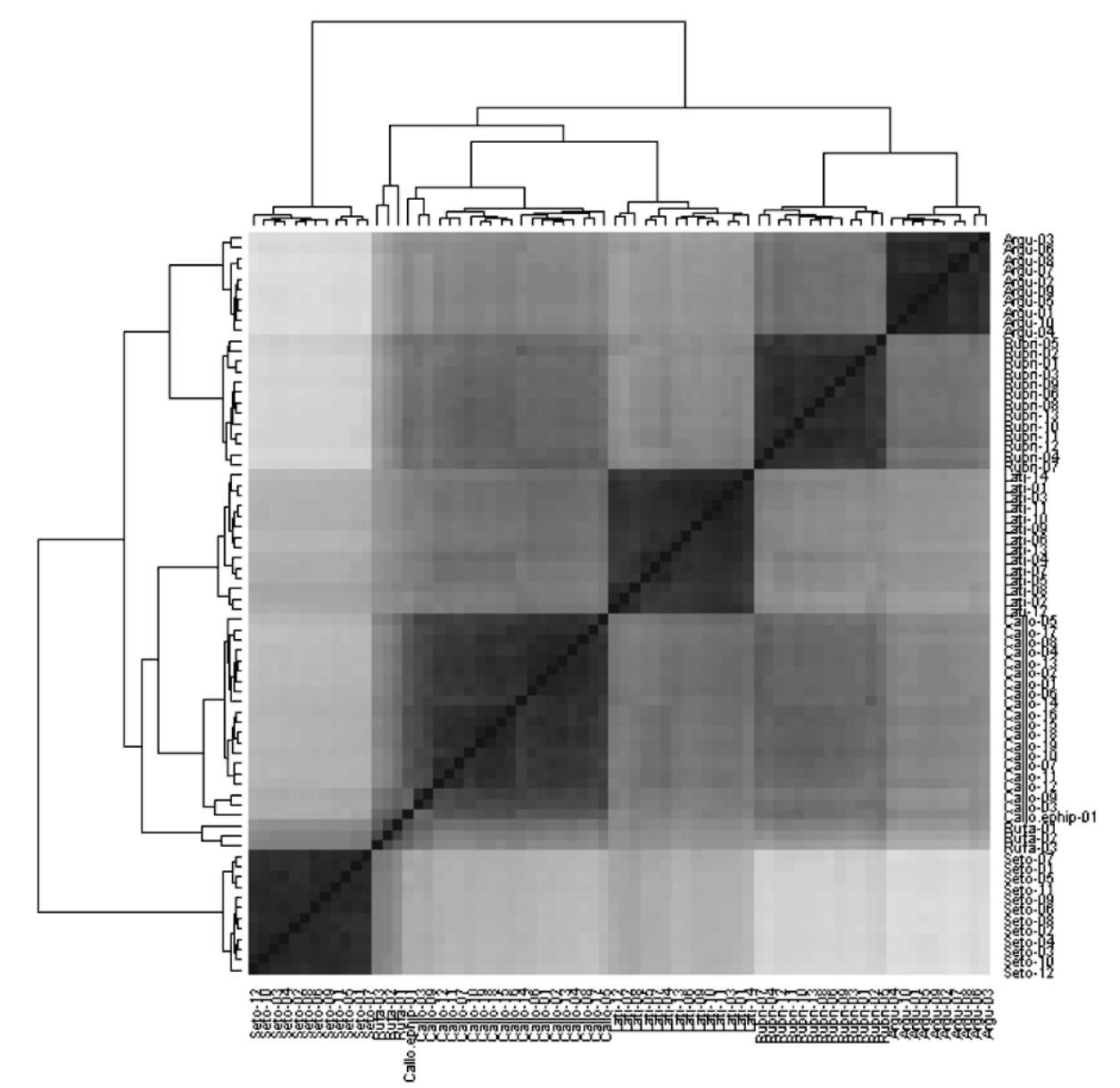
Figure 2 shows the phylogenetic relationships between Actinidia accessions in this study. From the phylogenetic tree, A. chinensis var. setosa and A. rufa in the FOT2 were combined to form a separate branch. Although all phylo-genetic relationships between A. chinensis var. setosa accessions were very consistent, those between accessions of A. rufa were highly variable. Actinidia arguta, A. latifolia, A. callosa var. calloa, A. callosa var. ephippioidea, and A. rubricaulis formed another highly diversed branch; the branch with the highest diversity was found between A. callosa accessions, and the most consistent relationships were between accessions of A. arguta. On all branches, A. callosa var. callosa and A. callosa var. ephippioidea showed the closest phylogenetic relationship, followed by A. chinensis var. setosa and A. rufa. The remaining species, A. latifolia, A. arguta, and A. rubricaulis, were located on more independent branches. Furthermore, there were no significant phylogenetic correlations between Actinidia accessions and geographic distribution.
Figure 3 shows the Q-Q type seriated heat map of Ac-tinidia accessions, where 72 accessions were divided into 5 observable groups. On the map, accessions of A. rufa and A. callosa var. ephippioidea were between accessions of A. callosa var. callosa and A. chinensis var. setosa; thus creating a slightly fuzzy boundary for the A. callosa var. callosa group. During our investigations A. rufa and A. callosa var. ephippioidea were found only in the areas where A. chinensis var. setosa and A. callosa var. cal-losa accessions overlapped. Comparing all characters of the four taxa, we found that most characters of A. callosa var. ephippioidea and A. rufa were intermediate between those of A. chinensis var. setosa and A. callosa var. cal-losa, creating a series gradient phenomenon. However, A. rufa has some traits that also belong to A. chinensis var. setosa, so it was combined into a separate branch with A. chinensis var. setosa on the phylogenetic tree. In contrast, in terms of overall similarity, A. rufa and A. callosa var. ephippioidea were closer to A. callosa var. callosa, not A. chinensis var. setosa, which affected the boundary of the A. callosa var. callosa group on the seriated heat map. A comparison of the characters, wild habitats, population locations, previous studies (cf. Mallet, 2007; Peng and Ku, 2009; Suezawa, 1989) and the results of the seriated heat map of the aforementioned four taxa suggests that A. rufa and A. callosa var. ephippioidea represent natural hybrids between A. chinensis var. setosa and A. callosa var. cal-losa, which show a one-way introgression hybridization trend toward A. callosa var. callosa (cf. Wiens, 2000).
Natural hybrids led to the incongruency between the phylogenetic tree and seriated heat map. Actinidia callosa var. callosa was closer to A. rubricaulis on the phyloge-netic tree, but was next to A. chinensis var. setosa on the heat map because of hybridization between Actinidia cal-losa var. callosa and A. chinensis var. setosa. This also led
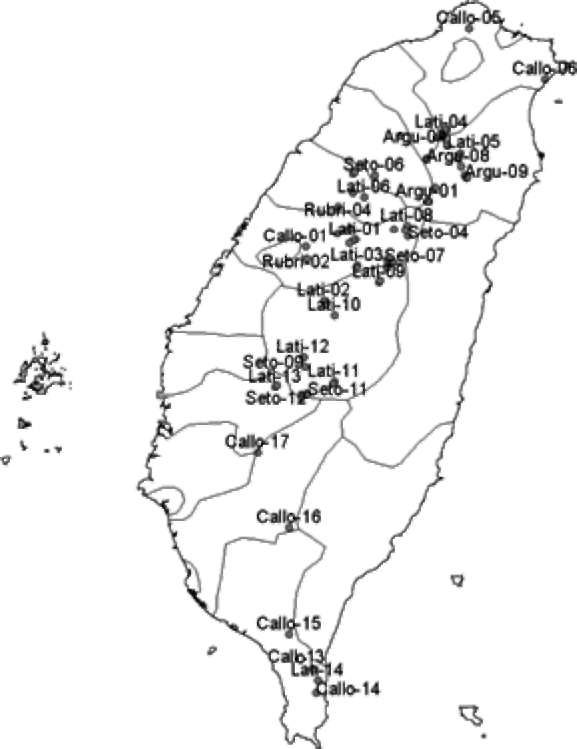
Figure 1. Distribution map of wild Actinidia accessions in this study. Some endangered accessions are not shown on this map. The code of accession names are based on identifications in the Flora of Taiwan 2nd ed. Lati-01-14 are A. latifolia. Callo-01-19 are A. callosa var. callosa. Argu-01-10 are A. arguta. Seto-01-12 are A. chinensis var. setosa. Rubri-01-13 are A. rubricaulis. Rufa-01-03 are A. rufa. Callo.ephip-01 is A. callosa var. ephip-pioidea.
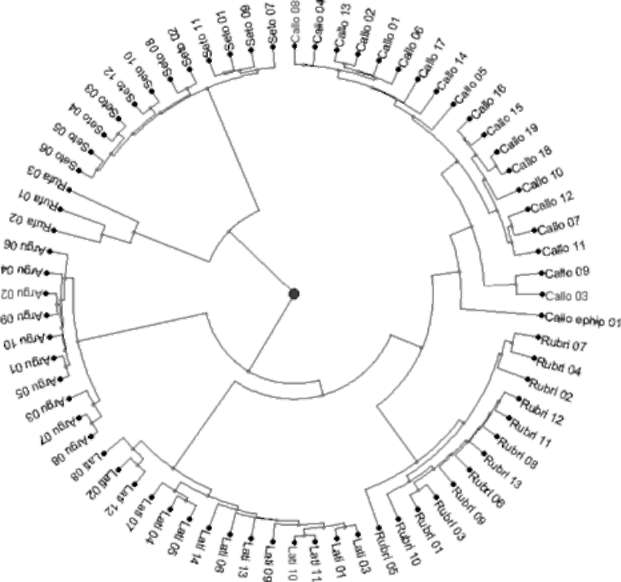
Figure 2. Phylogenetic tree of Actinidia accessions in Taiwan. Actinidia accessions in the phylogenetic tree were coded based on the abbreviations of specific epithets from the Flora of Taiwan 2nd ed.,