TAI et al. ― A one-time inducible transposon for terminating selectable markers in transgenic plants
377
into rice plants by use of transfection with Agrobacterium tumefaciens strain LBA4404. We used 21 single-copy T-DNA integration transgenic plants for the induction and detection of transposition events.
the endogenous rice epsps. This primer is mismatched at the two 3' terminal bases for endogenous epsps and has more power to identify transgenes. The total RNA of each transgenic line was extracted for RT-PCR. By using primers R-E1FC and mKRT2R, PCR reactions yielded single 439-bp DNA fragment (Figure 1B). These results indicated the normal expression of epsps gene. Additionally, since the transposase gene was triggered with PR-1a inducible promoter, the induction was carried out by applying 5 mM SA in the CIM medium for 7 days for single-copy trans-genic rice calli. By using primers CF and ER to determine the transposase transcripts, a 263-bp DNA fragment was obtained (Figure 1C). Sequencing analysis confirmed the normal transcripts of the epsps and transposase genes in COKC (data not shown). All these results indicate that the transposase and marker genes expressed normally in the transgenic plants.
Normal expression of transposase and marker genes with COKC
Previously, we created a construct by inserting the Ac 5' end into the first intron of a glyphosate-tolerate rice epsps gene to create a "marker-off" transgenic system (Charng et al., 2008). The expression of this modified epsps gene showed normal and additional transcripts. Besides the first intron of the modified epsps gene, transcription proceeded to the partial 5' end of the transposon, which resulted in an alternative splicing RNA fragment (Charng et al., unpublished results). In construct COKC, the 3' end of Ac was inserted in the first intron of epsps gene while 5' end was in the third intron of tansposase gene (Figure 1A). We therefore determine whether the transposase and epsps genes were expressed normally in the transgenic plants by RT-PCR. Since, the modified epsps in the COKC construct and endogenous rice epsps differ in 4 bp, primer mKRT2R was designed to rule out the the possibility of amplifying
Transposition events of COKC and termination of the glyphosate-tolerant epsps in transgenic rice
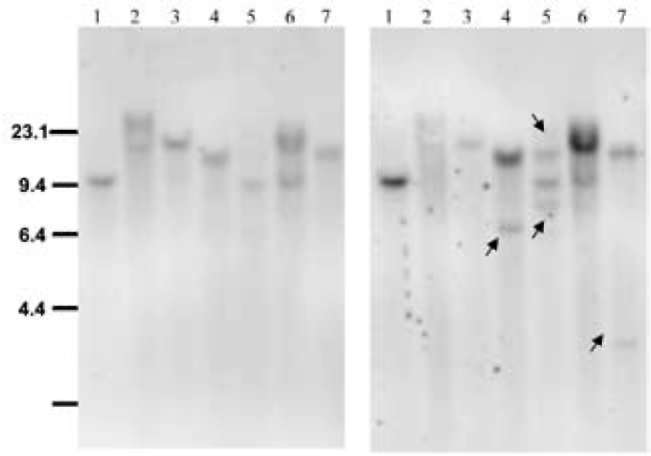
Previously, we introduced an Ac-based inducible trans-poson, INAc, into rice and found the highest transposition efficiency induced with 5 mM SA (Charng et al. 2007). Thus, to remove the functional glyphosate-tolerant epsps, we applied the same method trigger the COKC trans-poson. Calli regenerated from the T1 rice seeds of each transformed line harboring a single copy of COKC were incubated on callus induction medium containing 5 mM SA for 7 days then incubated on normal CIM for further regeneration. The excision events were determined by Southern blot and PCR analysis. Genomic DNA from 50 transformed lines was subjected to Southern blot analyses. As probes, the 1.4-kb Bam HI/Eco RV fragment comprising the HPT gene, and the PCR amplified fragment (comprising the 5' end, exon D and E of the transposase gene) were used. The DNA samples were digested with Spe I and hybridized with HPT probe. As an example, Figure 2 shows that the transformed lines 17, 19, 20, 21 and 24 yielded single hybridizing fragments demonstrating that these transformed line harboring a single copy of T-DNA. Single copy transgenic plants were confirmed together with further analysis, for example, progeny analysis (PCR based genotyping or glyphosate tolerance) and regular PCR to determine excision events and TAIL PCR to determine T-DNA/transposon flanking sequences (data not shown). Transformed lines 18 and 23 contain more than one T-DNA copies. After removal of the HPT probe, the same filter was hybridized with the TPase probe. The transformed lines 17, 18, 19, and 23 yielded the same hybridizing patterns, indicating the primary donor sites of the un-transposed COKC element. For transformed line 20, 21 and 24, in addition to the same hybridizing patterns with HPT probe (un-transposed COKC), several new bands were yielded indicating the transposition of COKC (Figure 2). By Southern blot analysis, 34 out of 50 transformed lines showed single copy of T-DNA integration (with HPT probe) and 4 out of these lines yielded new
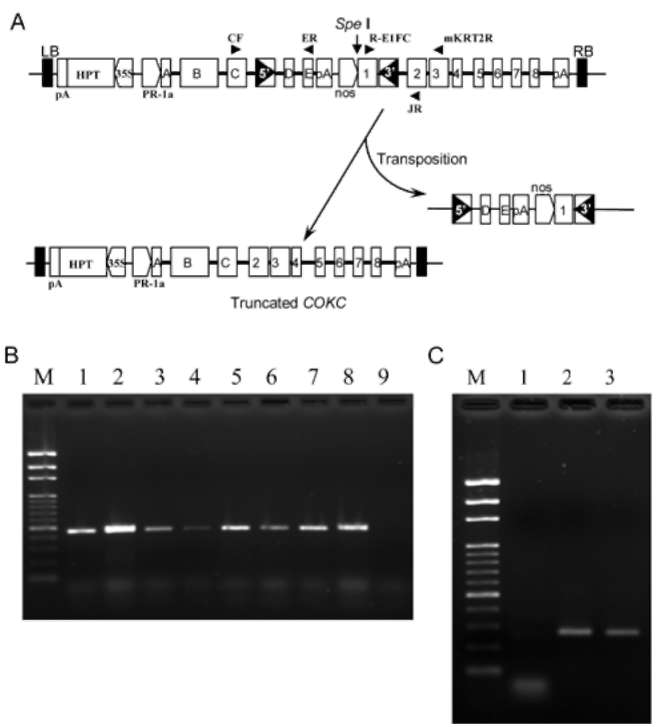
Figure 1. (A) Schematic diagram of the one-time transposon system COKC and location of primers (shown as solid triangle). LB, left border; RB, right border; 5' and 3', Ac left and right terminal-inverted repeat; PR-1a, PR-1a inducible promoter; HPT, hygromycin phosphotransferase gene; pA, poly(A) fragment; NOS, nopaline synthase promoter; A~E, transposase gene exon 1~exon 5; 1~8, epsps gene exons; (B) RT-PCR analysis of modified epsps expression in transgenic rice lines (1-8) and TNG67 (9); (C) RT-PCR analysis of induced transposase gene expression in transgenic rice calli treated with water (1) or 5 mM SA (2 and 3). M, 100 bp marker.