LU et al. ― Antibacterial and cytotoxic activities of wild bitter gourd
429
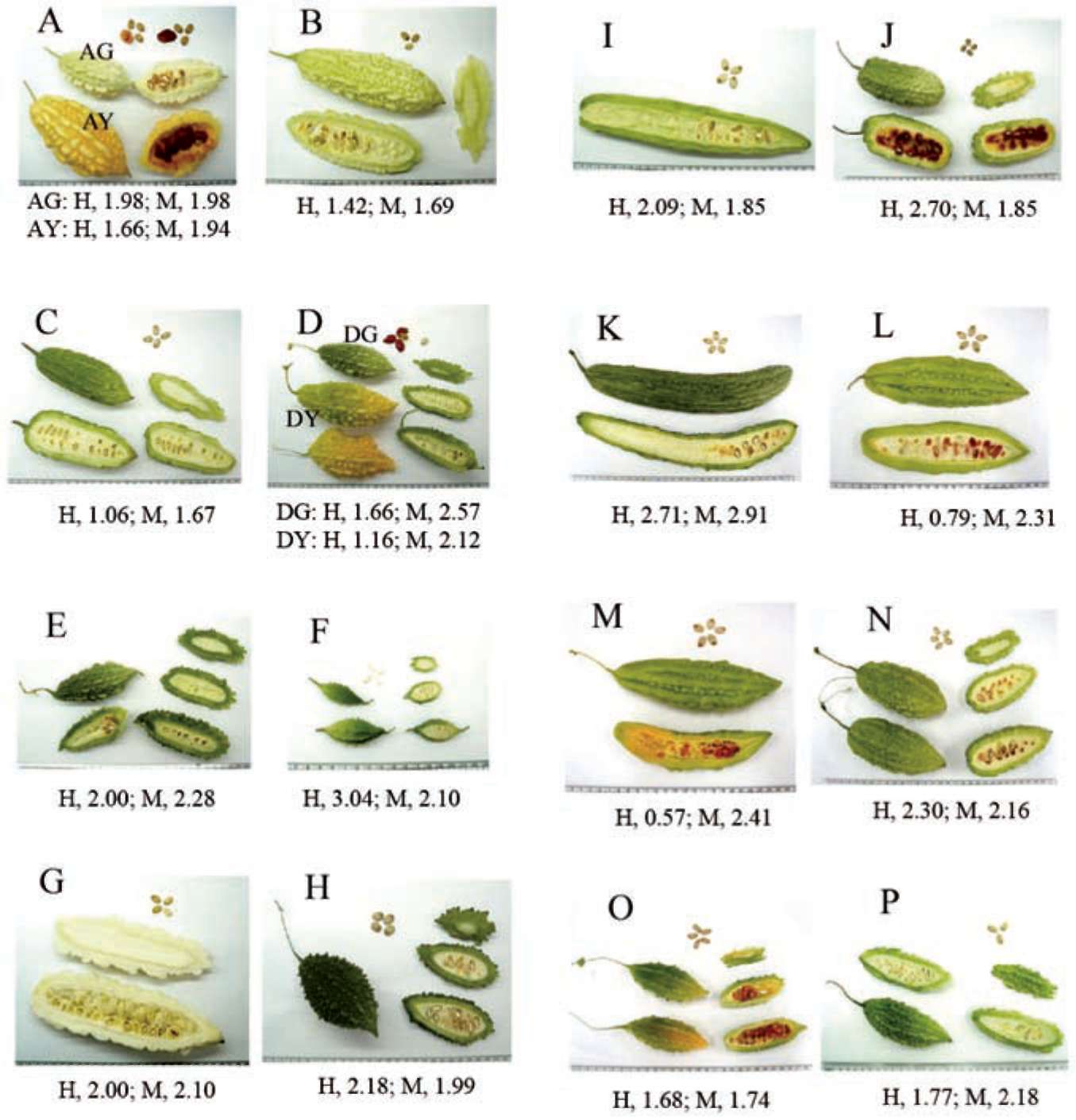
Figure 1. Photographs of different of wild bitter gourd (Momordica charantia L. var. abbreviata Seringe, MCA) cultivars used in experiments. There were sixteen cultivars (A to P) of MCA, cultivars of A and D contained the mature (AG and DG, green flesh) and ripe (AY and DY, yellow flesh) samples. The recovery (%) of each extract is shown at the bottom (H, water extracts; M, methanolic extracts).
cades, S. aureus (MRSA) has become a major nosocomial pathogen, and the therapeutic options for MRSA infection are very limited since most MRSA strains are resistant not only to p-lactams but also to multiple antimicrobial agents (Maple et al., 1989; Shiota et al., 1999). The p-lactam family of antibiotics includes many of the most commonly used antibacterials in clinical medicines. The majority of clinically useful p-lactams belong to either the penicillin (penam) or the cephalosporin (cepems) group (Tyc-
zkowska et al., 1994). One of the major mechanisms of resistance to p-lactams was the expression of p-lactamases which hydrolyzed the p-lactam ring. The p-lactamases (EC 3.5.2.6), such as penicillinase and cephalosporinase, which degraded penam and cepems, respectively, have been found widely in both gram-positive and gram-negative bacteria (Livermore, 1995). In order to inhibit food-borne pathogens and to extend shelf life, synthetic chemicals are often used as preservatives in food processing and storage.