LO et al. ― Structural and functional investigations on actin-like protein filaments in mung bean mitochondria
447
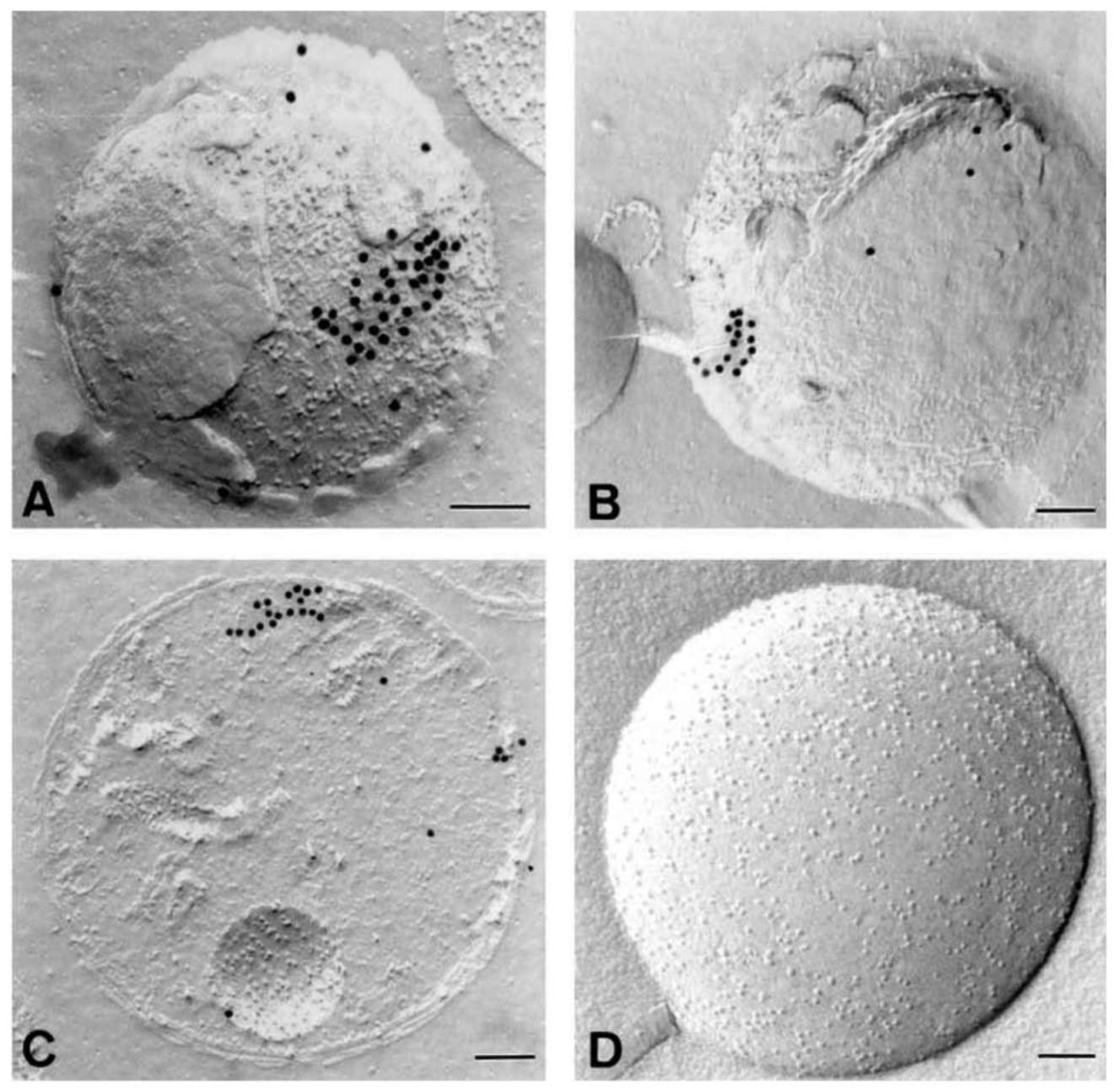
Immunogold labeling of anti-actin antibody in freeze-fractured mitochondria (Figure 2) indicates that a high density of gold particles accumulate as a patch and arranging in lines on protoplasmic surfaces of the mitochondrial membrane (Figure 2A). Since IMPs heavily cover this dislodged and exposed area, actin (or actin-like) filaments are likely attached to the mitochondrial inner membrane (Figure 2A). Figures 2B and 2C show that gold-labeled antibody reacted with actin or actin-like proteins located
under the mitochondrial outer membrane and in the matrix, respectively. In both cases, the linear arrangement of gold grains indicates the presence of actin or actin-like filaments with mitochondria. Figure 2D is the control of Figure 2A-C. Almost no cross reaction between the control antibody (PM 028) and the freeze-fractured mitochondria could be detected (Figure 2D and unpublished results).
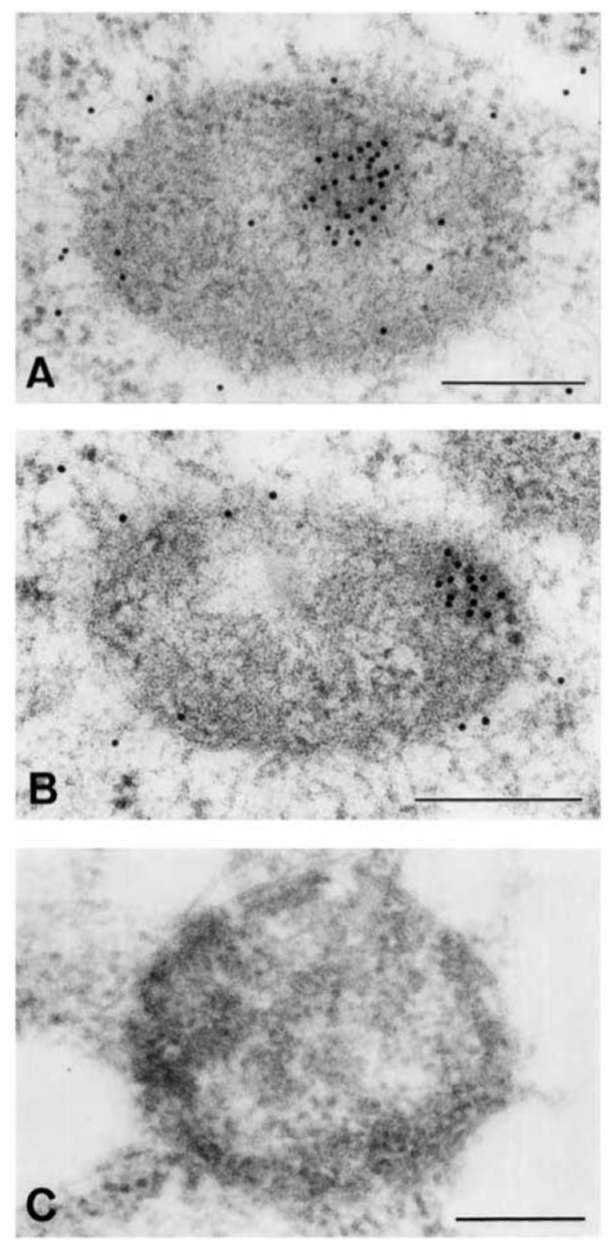
In situ immuno-gold labelling on thin sections of mung bean mitochondria revealed the localization of actin in the
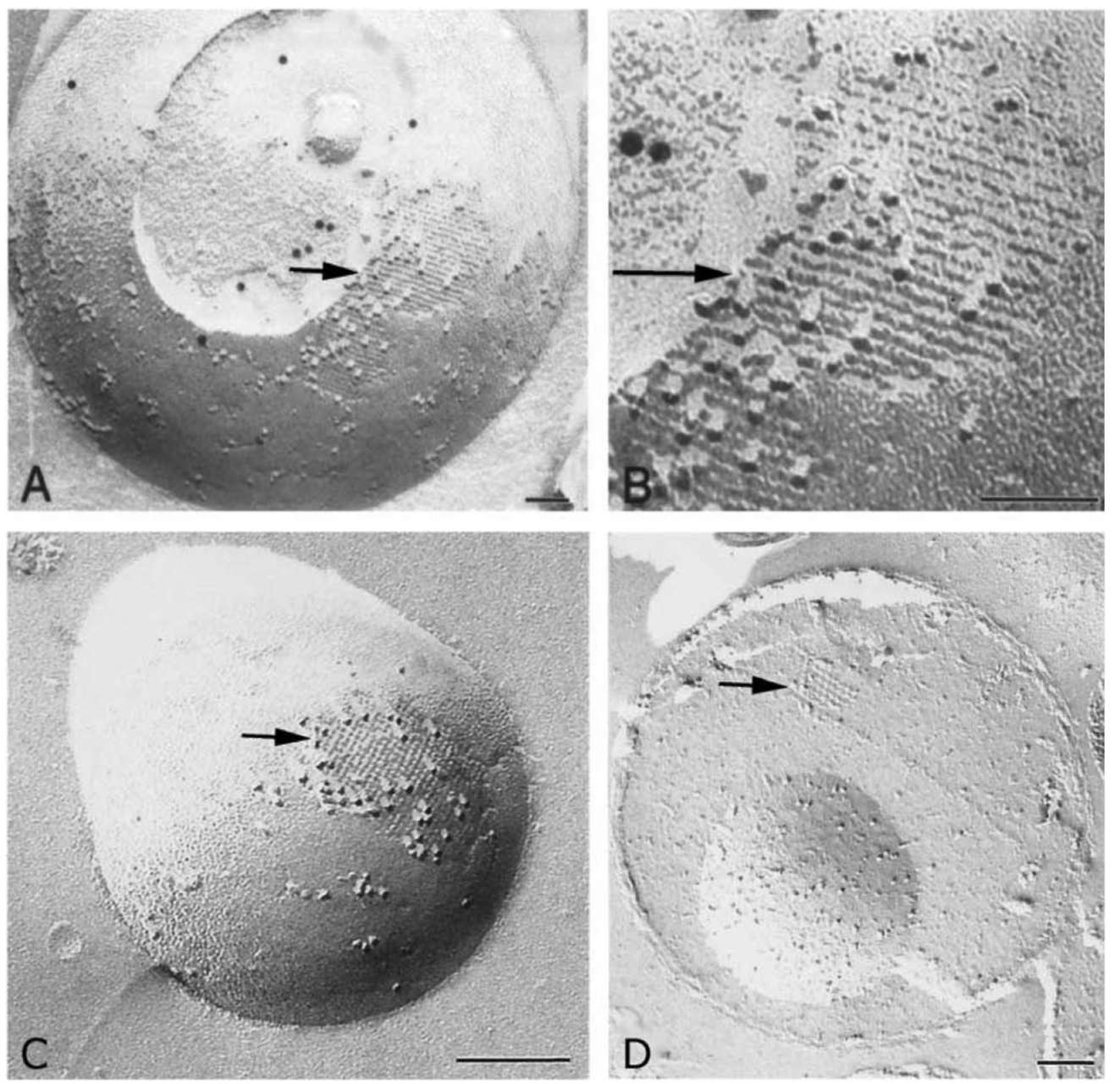
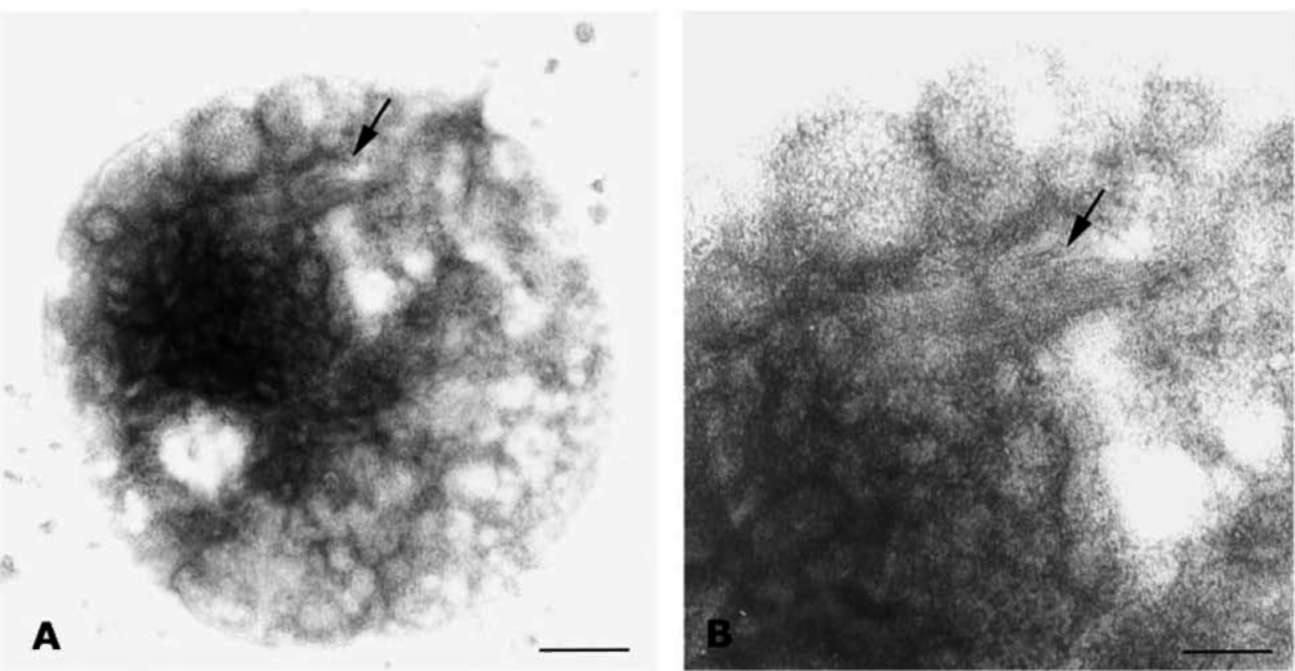
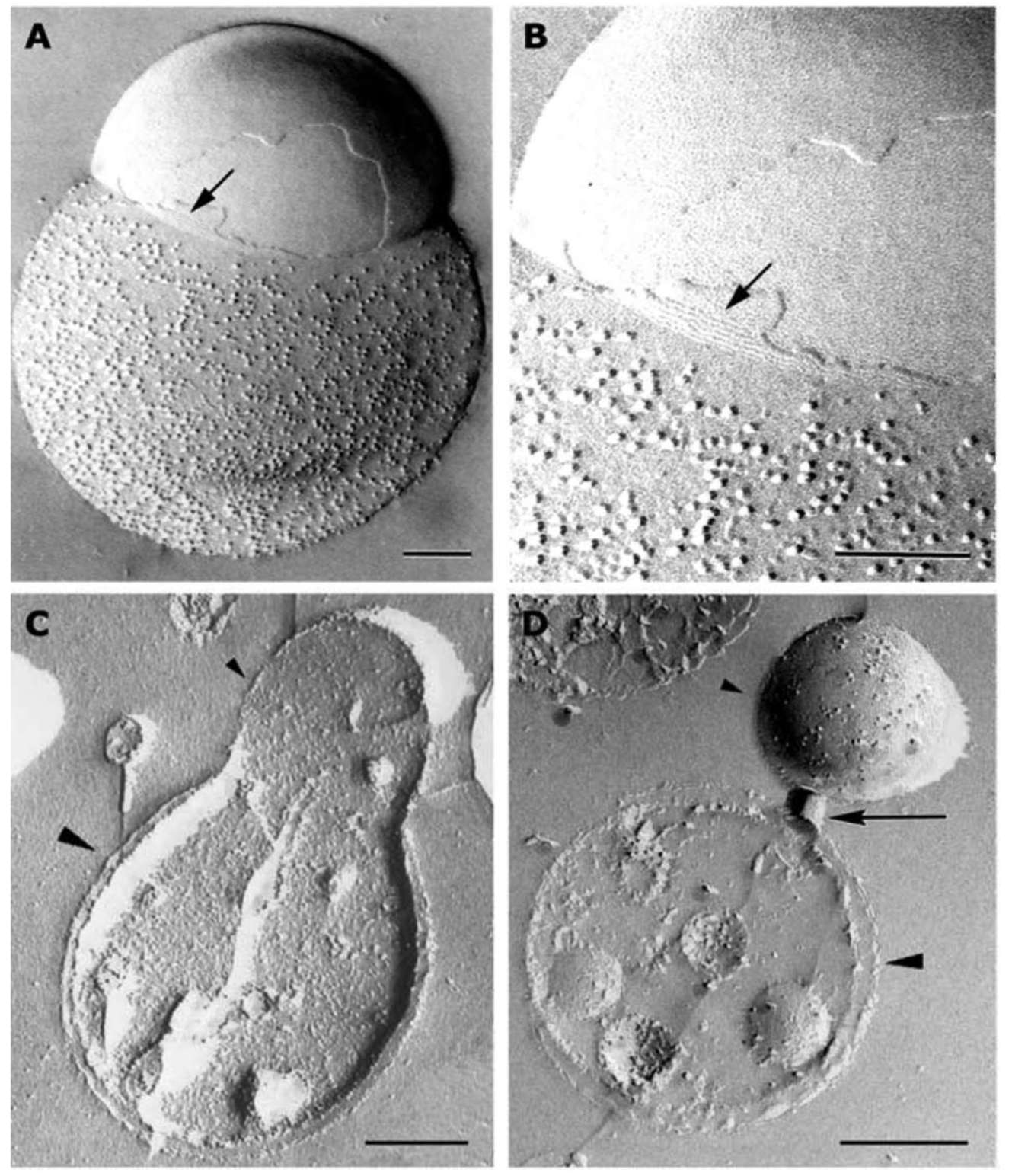
Figure 1. A 5 nm filamentous structure is visualized underneath the surface of mitochondria. Purified mitochondria were subjected to freeze-fracturing, and then immunogold labeling using anti-COX III antibody (for A and B) after laying the metal replica (see Materials and Methods). The arrow points to a fractured area in which the mitochondrial outer membrane appears dislodged, exposing the intramembranous particles (IMPs). The 5 nm-wide filaments lie adjacent to and just below these IMPs. Note that the width of the filaments and their constituting subunits display a good degree of uniformity and periodicity. The area indicated by the arrow in Panel A is amplified in Panel B. Panels C and D show a 5 nm filamentous structure with cross meshwork arrangements. Panel C shows filaments under the surface of mitochondrion as in Panel A. Panel D indicates cross meshwork and filament structure in mitochondrial matrix. All filaments are composed of globular protein. Bar equals 200 nm.