458
Botanical Studies, Vol. 52, 2011
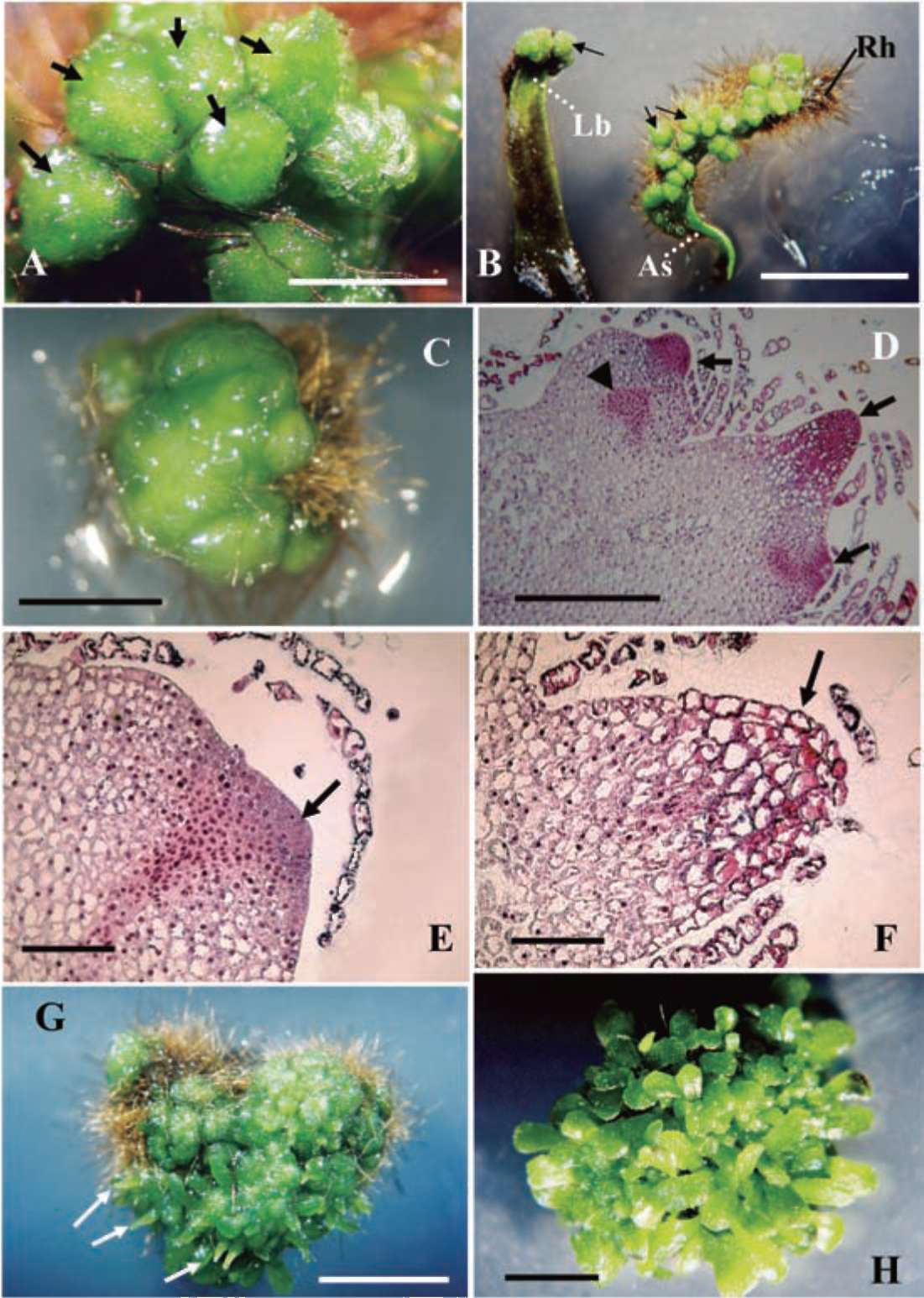
Figure 1. Microscopic examination of tissue-cultured Platycerium bifurcatum via GGB initiation and multiplication (A-D), and subsequent stages of sporophyte regeneration (E-H). (A) A close-up view of a GGB cluster cultured on MS medium supplemented with 5.37 (M NAA after 4 weeks; each GGB (arrows) exhibits a green glassy bead-like shape (bar = 0.1 cm); (B) GGBs (arrows), surrounded by rhizoids (Rh), mainly initiated from the adaxial surface (As) or leaf base (Lb) of the proximal explant sections (bar = 0.5 cm); (C) Single GGB cultured on MS medium containing 2.22 (M 2ip and 5.37 (M NAA after 3 weeks. Multiplication of small nodule-like structures made them difficult to separate at this stage (bar = 0.25 cm); (D) GGB tissues showing multiple meristematic areas with dividing cells distributed internally (triangular marker) or around the nodule surface (arrows) (bar = 0.5 mm); (E) The initial stage of sporophyte formation occurred due to the high division of cells on the epidermis of the GGB tissue (arrow) (bar = 0.2 mm); (F) Subsequent elongation of initial sporophyte (arrow) from the GGB surface (bar = 0.2 mm); (G) Light green elongated sporophyte (arrows) becomes visible. Stereomicroscopic examination shows straightforward growth out of the GGB tissue (bar = 0.5 cm); (H) Regenerated sporophytes with fully extended leaves grown on the GGB tissue (bar = 0.5 cm).
