466
Botanical Studies, Vol. 52, 2011
the function of LEA proteins. Cryoprotection or desiccation protection assays showed that several LEA proteins maintained the function of stress-sensitive enzymes after freezing or dehydration (e.g., Kazuoka and Oeda, 1994; Honjoh et al., 2000; Goyal et al., 2005; Grelet et al., 2005; Nakayama et al., 2008). FTIR analysis results indicated that LEA proteins increased the glass transition temperature (Tg) of non-reducing sugars and reduced the phase transition temperature (Tm) of phospholipids in the dehydrating condition (Wolkers et al., 2001; Shih et al., 2004;
2010a; 2010c).
Using 4-day pod-dried (PD) soybean seeds at 35 days post-flowering as material, we prepared a cDNA library (Hsing et al., 1990; Hsmg and Wu, 1992) and identified 41 cDNA clones designated Glycine max physiologically mature (GmPM). Twenty-two of them belonged to five groups of LEA genes. Molecular biology, expression profiling, or structural biology was used to reveal the functions of these proteins (e.g., Hsing et al., 1995; Lee et al., 2000; Shih et al., 2004; 2010a; 2010b; 2010c; Tsai et al., 2008). Most of the soybean LEA proteins studied accumulated in precociously or naturally matured seeds. Hydrophilic LEA proteins (groups I to IV) were members of natively unfolded proteins or intrinsically disordered proteins, and the proteins can change their conformation and interact with macromolecules, such as non-reducing sugars, phospho-lipids, or polypeptides.
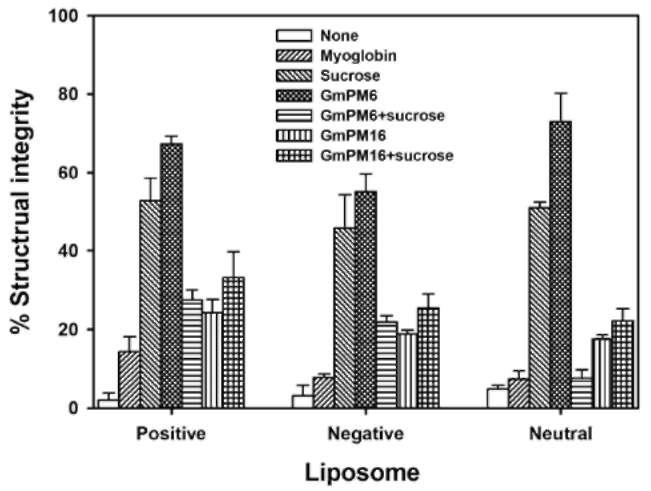
Here, we present the results of desiccation protection assay of two hydrophilic soybean LEA proteins, GmPM6 (LEA II, 23.7 kDa, pI value 6.1) (Soulages et al., 2003; Shih et al., 2010c) and GmPM16 (LEA IV, 14.7, pI value 9.7) (Shih et al., 2004) by monitoring the integrity of lipo-somes after desiccation. We discuss the possible role of the LEA proteins in desiccation and dry storage tolerance.
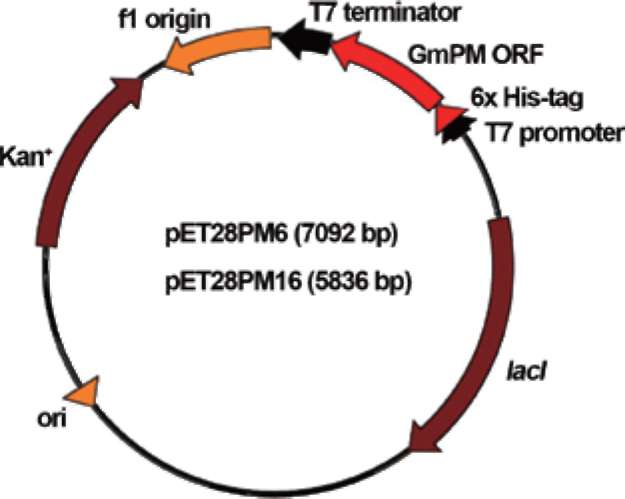
Figure 1. Scheme of construction of the GmPM6 and GmPM16 fusion plasmids.
1 min, with a final extension at 72°C for 5 min. The amplified PCR products were separated and purified by 1% (w/v) agarose gel electrophoresis. Products were digested with the restriction enzymes Ndel and Xhol, then ligated into the pET28a T7 expression vector (Novagen), which had been pre-cut with the same enzymes (Figure 1). The recombinant plasmids were introduced into E. coli cells, and the expression of the recombinant genes was enhanced by 1 mM IPTG induction. Recombinant proteins were purified by an immobilized-metal affinity column as per the manufacturer's protocol (pET system manual ver. 5.0, Novagen, Germany). Purified recombinant proteins were examined by one-dimensional 12.5% SDS-polyacrylamide gel electrophoresis, followed by Coomassie blue staining.
MATERIALS AND METHODS
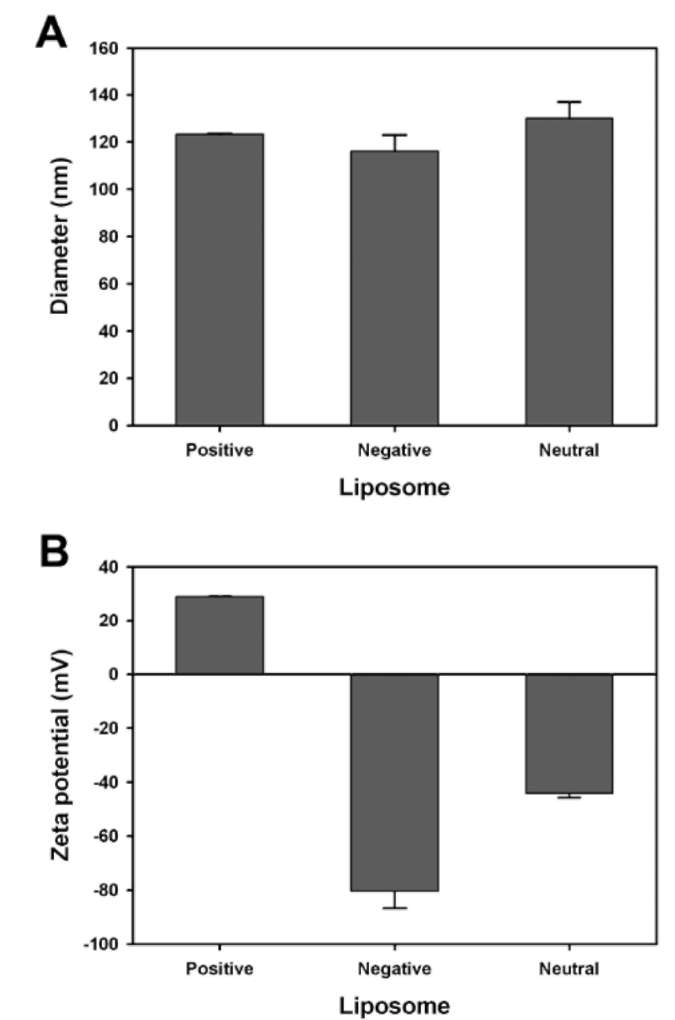
Liposome preparation
Bacterial strains and media
Myoglobin (17.2 kDa, pI value 7.1), 5(6)-carboxy-fluorescein (CF), and sucrose were from Sigma (USA). 1-palmitoyl-2-oleoyl-3-sn-phosphatidylcholine (POPC, 760 kDa) was from Avanti Polar Lipids (USA). Liposomes containing CF in Tris buffer were prepared by reverse-phase evaporation (Szoka and Papahadjopoulos, 1978). Five micrograms of POPC was dissolved in organic solvent into which 5 mol% dicetyl phosphate or 5 mol% stearylamine was added to give an overall negative or positive charge. Neutral liposomes were made from POPC. The solvent was removed under reduced pressure at 35°C on a rotary evaporator. The dried lipids were dissolved in 1 ml of 1 mM Tris, pH 8.0, containing 150 mM CF. The tube was sonicated in a bath-type sonicator for 10 min at 30°C until a uniform emulsion formed. Liposome suspensions (multi-lamellar vesicles) were sonicated by use of a probe-type sonicator. Sonication was carried out intermittently at 5°C for 5 min. After sonication, unincorporated mate-rial was removed by passing the large unilamellar vesicles through a Sephadex G-50 column equilibrated with 1 mM Tris, pH 8.0, to stabilize the LEA proteins.
Escherichia coli strains XL1-Blue and BL21(DE3) were used as the host. XLl-Blue was used for cloning plasmids: and BL21(DE3) was used for expressing recombinant proteins and growth analysis. All cultures were grown in LB-medium in the presence of 50 fig/ml kanamycin at 37°C.
Recombinant plasmid construction, expression, and purification of its products
Recombinant DNA techniques were performed es-sentially as described (Sambrook and Russell, 2001). The NdeI-XhoI fragment of GmPM6 or GmPM16 containing the coding region was amplified by specific forward and reverse primers (GmPM6: 5'-ggttgacgCATATGgcaacgt-tg-3' and 5'-catgcatg-
CTCGAGgcacgatgc-3'; GmPM16: 5'-gaagaaca-
CATATGcaatcctc-3' and 5'-gacgtactCTCGA-Gaaacacag-3')
from maintenance plasmids harboring the full-length cDNA of the GmPM clones. The PCR protocol was an initial denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 45 sec, 55°C for 30 sec and 72°C for
CTCGAGgcacgatgc-3'; GmPM16: 5'-gaagaaca-
CATATGcaatcctc-3' and 5'-gacgtactCTCGA-Gaaacacag-3')
from maintenance plasmids harboring the full-length cDNA of the GmPM clones. The PCR protocol was an initial denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 45 sec, 55°C for 30 sec and 72°C for