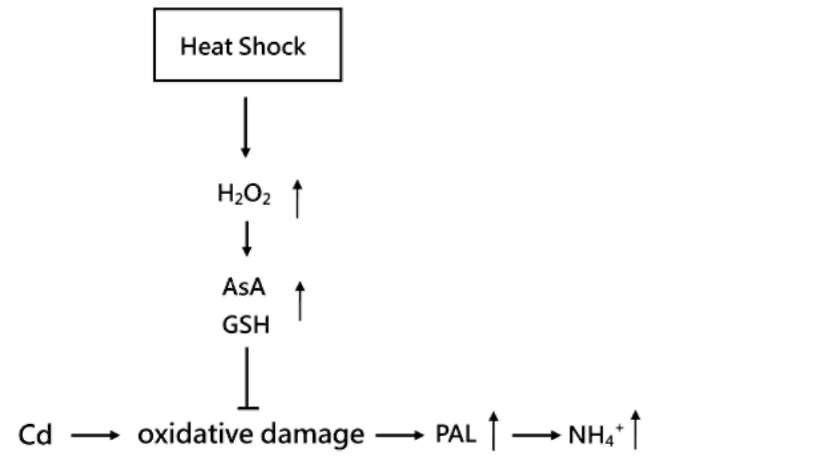
KUO et al. — Cd effects in rice seedlings after heat shock
473
solution with or without 5 j M CdCl2 for 6 days. The Cd concentration in shoots and roots of rice seedlings without Cd was 0.64 士 0.24 for shoots and 0.78 士 0.09 jag g-1DW for roots. We observed a marked increase in Cd concentration in Cd-treated shoots and roots (5.89 士 0.7 for shoots and 11.8 士 1.7 jag g-1DW for roots).
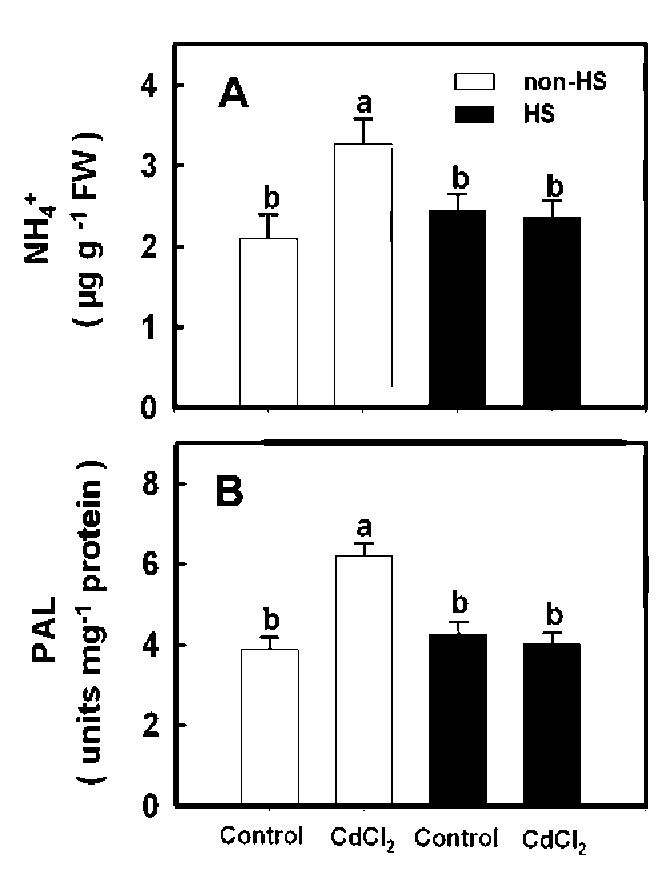
Changes in NH4+ content and PAL activity during Cd stress
To examine the changes in the content of NH4+ and the activity of PAL during Cd stress, rice seedlings were grown in nutrient solution with or without 5 j M CdCl2. We observed an increase in the content of NH4+ six days after treatment and in the PAL activity caused by CdCl2 four days after treatment (Figure 1A and 1B).
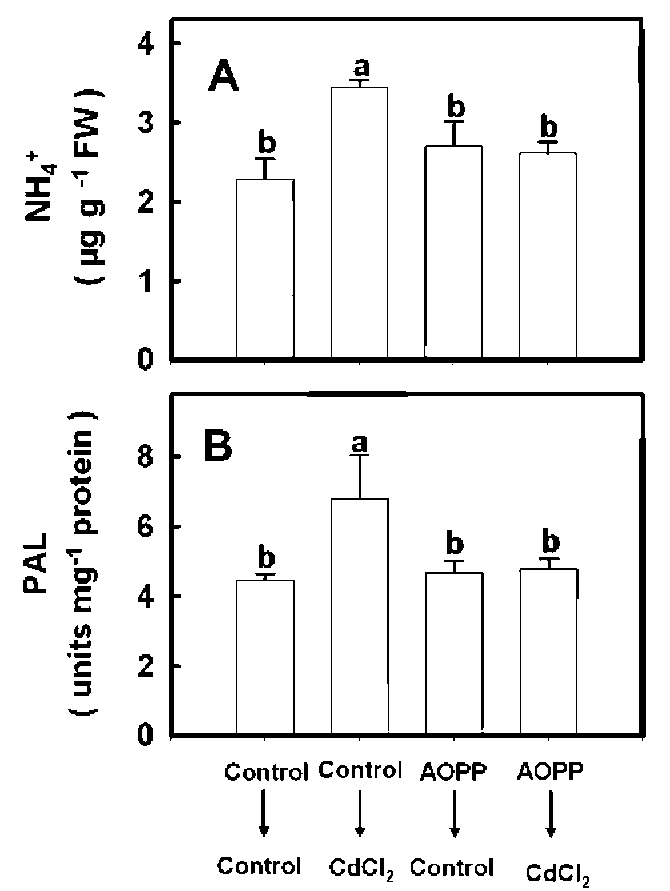
Effects of a-aminooxy-6-phenylpropionic acid
a-Aminooxy-p-phenylpropionic acid (AOPP) is a potent PAL inhibitor (Amrhein and Godeke, 1977). To examine its effect on Cd-increased PAL activity and NH4+ content in leaves, rice seedlings were first pretreated with or without 25 j M AOPP for 3 h and then transferred to nutrient solution with or without CdCl2 for six days. We observed that Cd-increased PAL activity and NH4+ content in leaves were significantly inhibited by AOPP (Figure 2).
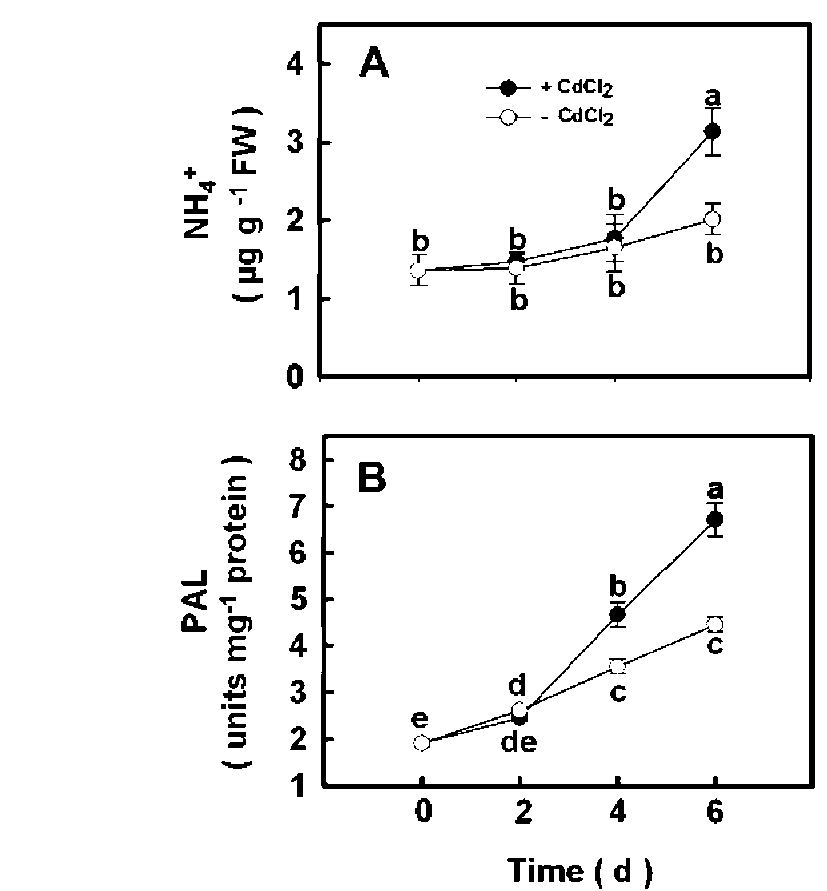
Figure 2. Effect of CdC^ on the content of NH4+ (A) and the activity of PAL (B) in the second leaves of rice seedlings pre-treated with or without 25 j M AOPP. Measurements were made 6 d after 5 jaM CdC^ treatment. Bars show means 士 SE (n = 4). Values with the same letter are not significantly different at
P<0.05.
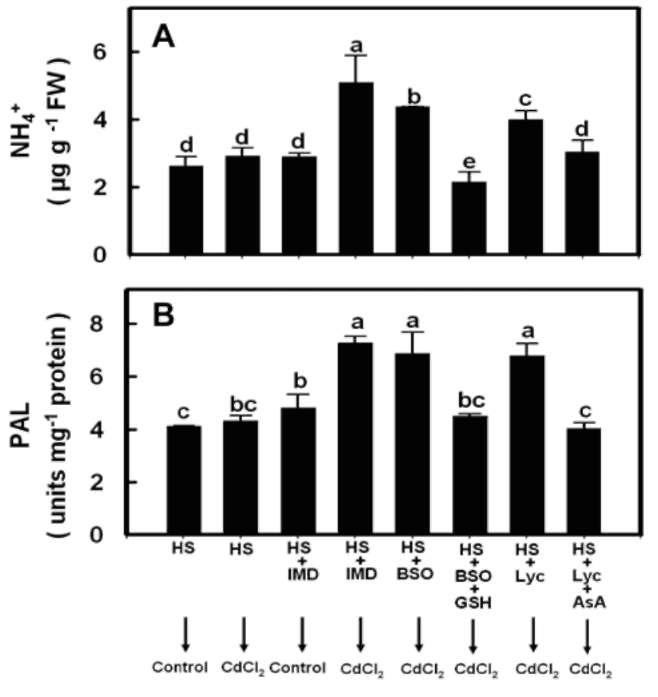
Effects of prior HS exposure
To test whether prior rice seedling exposure to HS affects the subsequent Cd-induced increases in NH4+ content and PAL activity in leaves, seedlings were pretreated with HS for 3 h under dark conditions. We observed that a 3 h HS pretreatment reduced both subsequent Cd-induced increases in NH4+ content and the PAL activity in rice seedling leaves (Figure 3A and 3B).
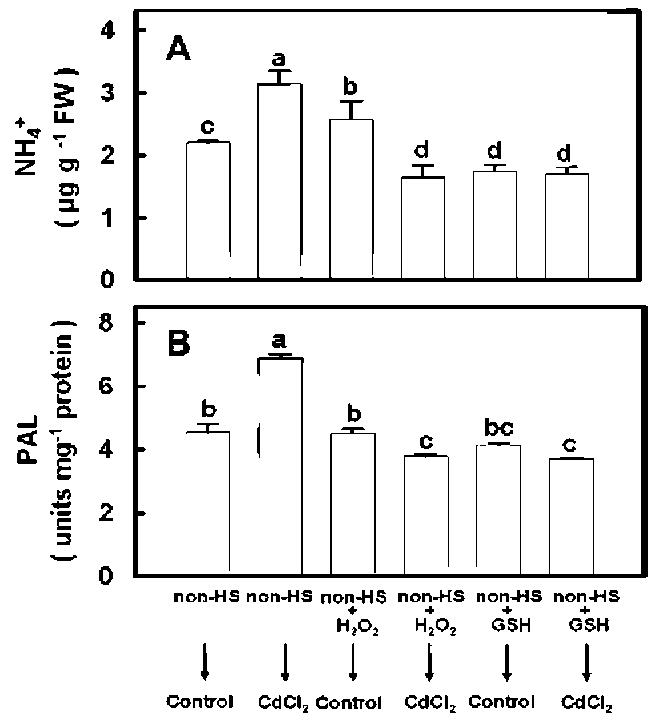
Pretreatment with H2O2 under non-HS conditions
We reported previously that H2O2 content increased in rice seedling leaves after HS exposure (Hsu and Kao, 2007). In view of this result, we studied the effect of H2O2 pretreatment under non-HS conditions on Cd-induced NH4+ content and PAL activity increases in leaves. To do this, rice seedlings were first pretreated with 0.1 mM H2O2 for 3 h under non-HS conditions, then transferred to a nutrient solution with or without CdCl2 for six days. We observed that pretreating rice seedlings with H2O2 reduced both the Cd-induced NH4 + content and PAL activity increases in leaves (Figure 4A and 4B).
Figure 1. Changes in the content of NH4+ (A) and PAL activity (B) in the second leaves of rice seedlings treated with or without 5 jaM CdCl2. Bars show means 士 SE (n = 4). Values with the same letter are not significantly different at P<0.05.