480
Botanical Studies, Vol. 52, 2011
rather than truly androdioecious (Charlesworth, 1984; Pannell, 2002). However, such studies are very limited and require careful examination on the functionality of the flowers. One of the candidates showing androdioecy came from the genus Symplocos (Symplocaceae), found during our previous plant survey of Taiwan (Tseng et al., 2008). Symplocaceae, a family in the order Ericales, consists of about 300 tree or shrub species, occurring in humid tropical or subtropical montane forests in eastern and southern Asia, America and Australia (Heywood et al., 2007; APG III, 2009). The family has been classified into two genera (genus Symplocos and Cordyloblaste) and several taxa above the species level (two subgenera, three sections, and two series in Symplocos; Fritsch et al., 2008).
The breeding system of the genus Symplocos is pre-dominately hermaphroditic (Heywood et al., 2007), but a few species show gender dimorphism (Aranha Filho et al., 2007, 2009a, b). Recently Aranha Filho et al. (2009a) doc-umented prevalent type I cryptic dioecy in Symplocos sec-tion Hopea, which is mainly distributed in South America (Fritsch et al., 2008; Aranha Filho et al., 2010). Many of these are morphologically androdioecious and at least 10 species examined by Aranha Filho et al. (2009a) produced sterile and malformed pollen grains on morphologically hermaphroditic flowers.
The only Asian representative of S. section Hopea is Symplocos wikstroemiifolia Hayata (Fritsch et al., 2008; Aranha Filho et al., 2009a). This species has been considered "probably androdioecious" (Wu and Nooteboom, 1996). Nagamasu (1998) described S. wikstroemiifolia as having "bisexual (female)" flowers in the 2nd edition of the Flora of Taiwan. Wang (2000) also stated that the stamens of morphologically hermaphroditic flowers are "undeveloped" in S. wikstroemiifolia. Although no detailed description exists in the literature, this species seems likely cryptically dioecious.
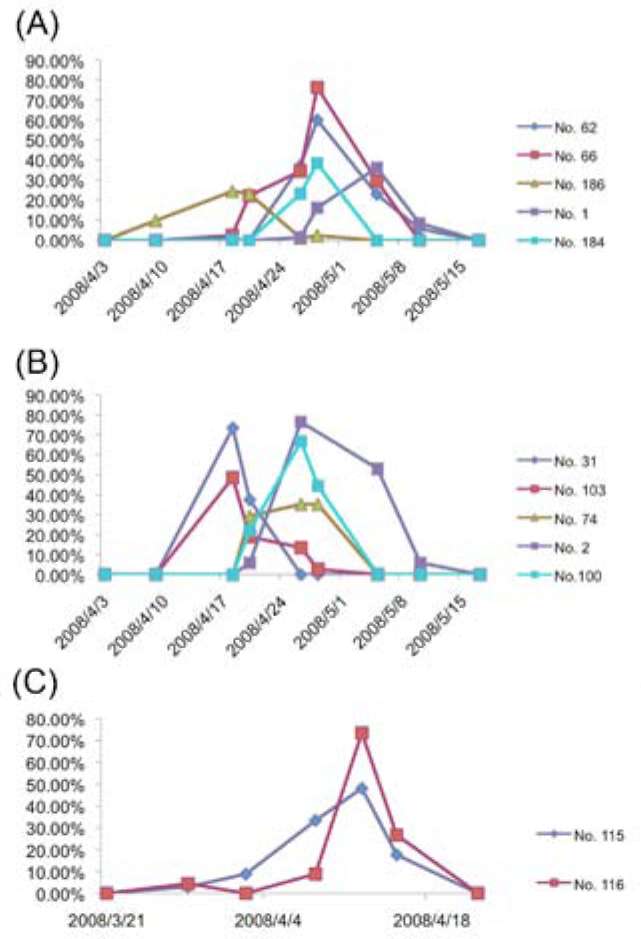
In this paper, we provide evidence for the functionality of the flowers in S. wikstroemiifolia, and also basic descriptions of the reproductive biology of this species in Taiwan. Sexual expressions of every flowering individual from two natural populations in Taiwan were recorded for at least two consecutive years. Flower morphology, phenology and natural fruit set were also investigated.
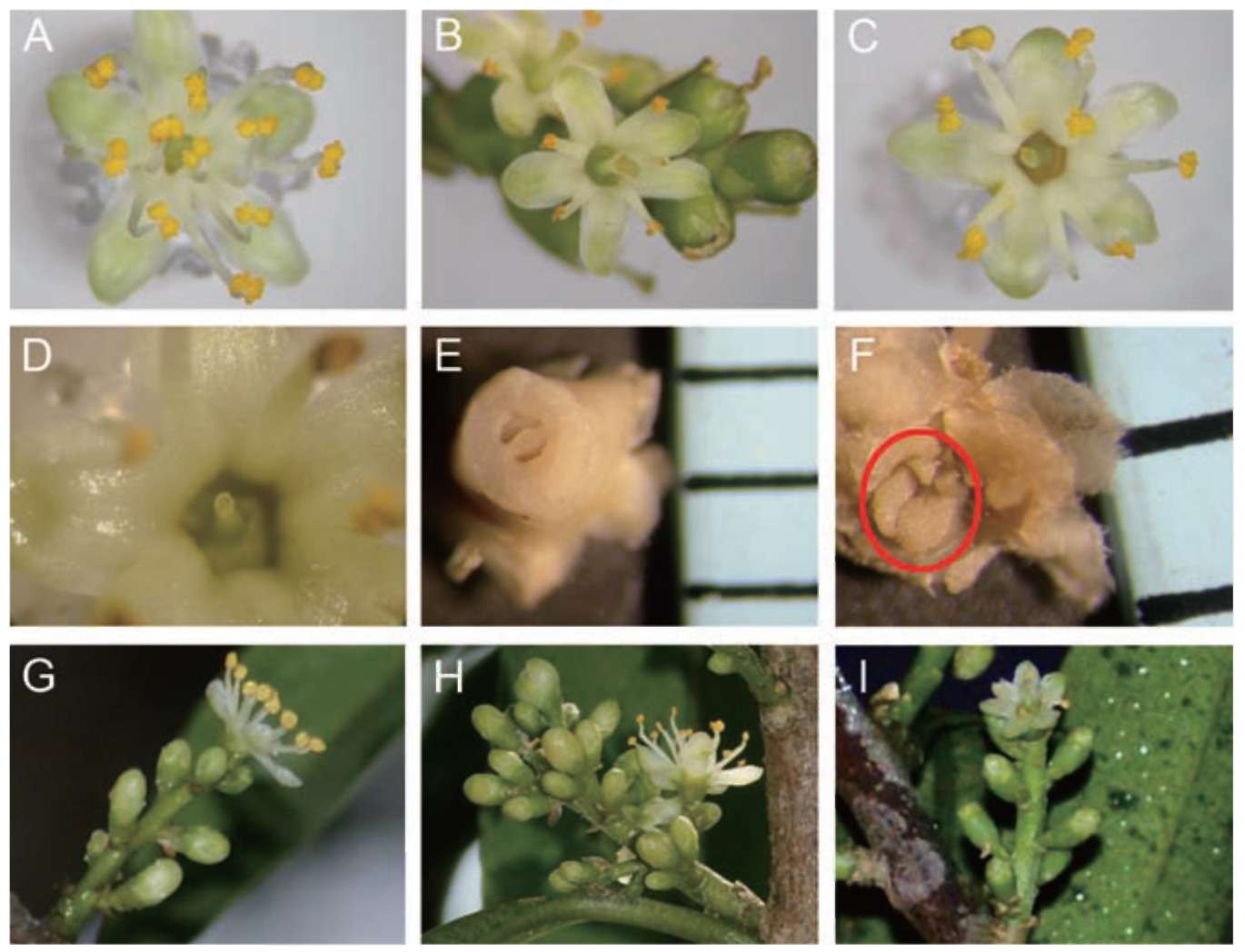
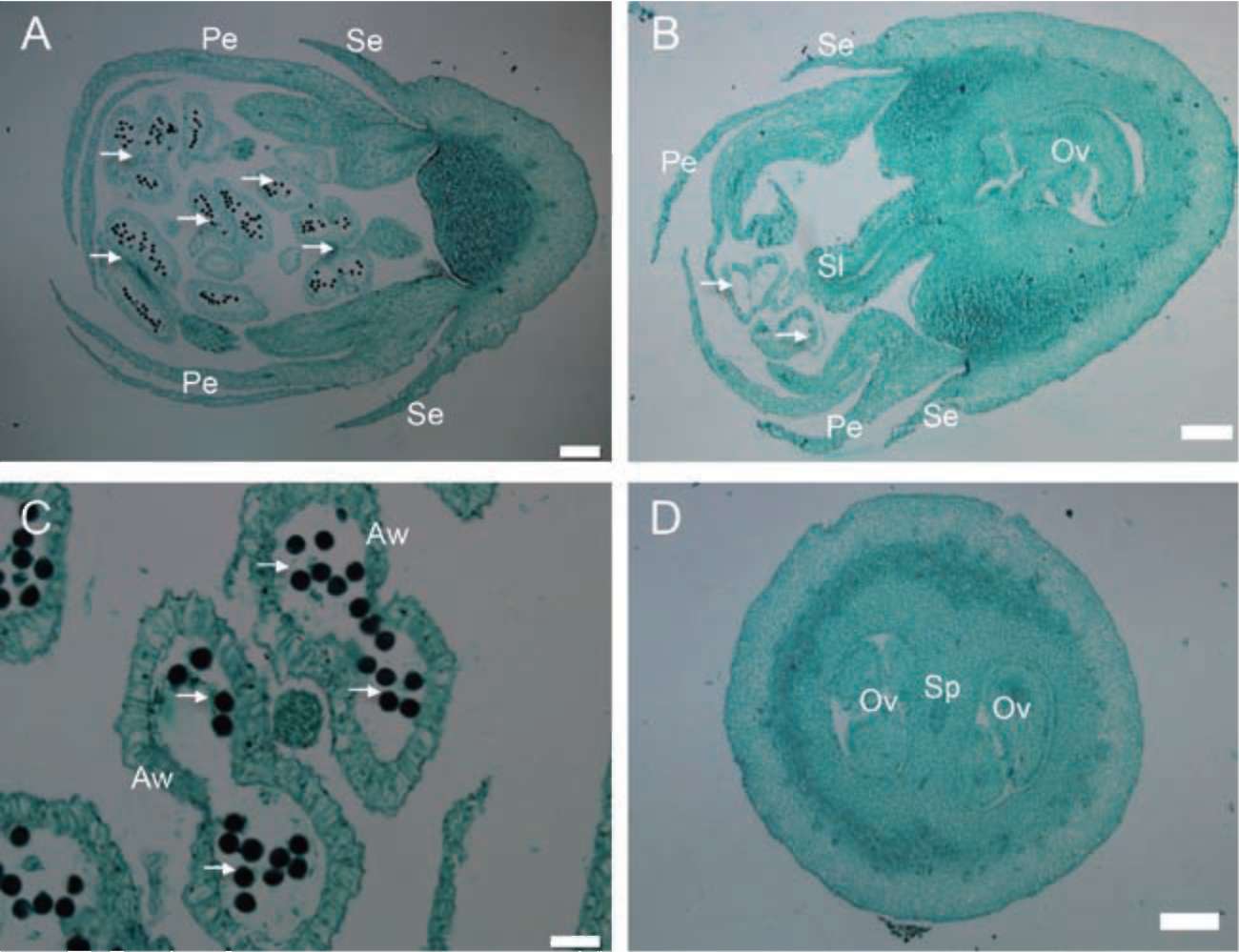
ovary. There is one style on the morphologically hermaphroditic flower but none on the male flower, and only one seed per drupe when mature.
Symplocos wikstroemiifolia is distributed in southern China, Indochina, the Malay Peninsula, and Taiwan (Na-gamasu, 1998). In Taiwan, the species is scattered across the middle and northern part of the island, from 700 m to 1,900 m altitude. They are not very common, and only locally abundant around Beichatian Mountain in Taiwan (Wang, 2000). Individuals start flowering from March to May, and set fruit from July to September (Wang, 2000).
Study sites
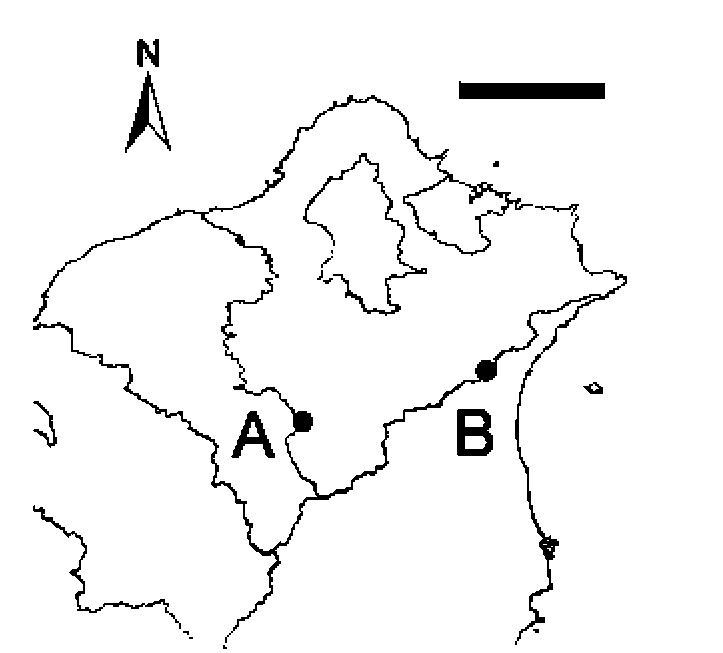
Field investigations were conducted at two sites where natural populations of S. wikstroemiifolia occur. One is located in the Chatianshan Nature Reserve, on the ridge around Beichatian Mountain (Figure 1). This area is one of the few places in Taiwan for Fagus hayatae (Fagaceae) (Liu and Su, 1972; Hsieh, 1989; Chiou et al., 1998; Lu et al., 1998). Beichatian Mountain is 1728 m high and S. wikstroemiifolia is particularly abundant at altitudes above 1,400 m on the surrounding ridge. The mean annual temperature is approximately 14.2°C (Chiou et al., 2004) and the mean annual precipitation, from 2005 to 2008, was 4,238 mm, as recorded by the Fushan Weather Station (about 5 km east of Beichatian Mountain and at 455 m altitude).
The other study site was the mountainous area of Pin-glin District, New Taipei City (Figure 1). This site is along the roadside of Provincial Highway No. 9 near the "54.5 K" road sign located in a valley near the Sidu River at about 400 m altitude. The mean annual temperature, from
MATERIALS AND METHODS
Study species
Symplocos wikstroemiifolia Hayata is an evergreen shrub or tree with grayish or brownish bark. The leaves are simple, alternate, with an entire or sometimes a crenu-late margin (Wang, 2000). This species is morphologically androdioecious (Wu and Nooteboom, 1996; Nagamasu, 1998; Wang, 2000). The male flowers usually have more than 10 stamens, whereas the morphologically hermaphroditic flowers have only five stamens. Both flower types are white, small and inconspicuous. They both have a five-lobed corolla and a nectary disk on the superior part of the
Figure 1. Study sites. Label A indicates the location of Beicha-tian Mountain. Label B indicates the second study site "Pinglin". The image is a magnification of a region from northern Taiwan. Scale bar = 25 km.