494
Botanical Studies, Vol. 52, 2011
vation and restoration of this species and its habitats are imperative and urgent (Pan and Zhang, 2002; Zhang et al., 2006). A general understanding of the ecology of this species and its community is crucial for its conservation and restoration. This study is about the ecological characteristics of G. uralensis and its communities. Although recent research has been conducted on the taxonomy, biochemistry, pharmacology and genetics of this species (Hu and Shen, 1995; Zhang et al., 2001), only limited research has been conducted concerning its ecology.
Our study mainly focuses on G. uralensis ecology with the objectives of: (1) identifying the communities of G. uralensis and analyzing their composition and structure; (2) elucidating the relationships between vegetation and environmental variables to find the most important ones between endangered species and their communities; and finally, (3) discussing some measures of conservation management for this species and its communities.
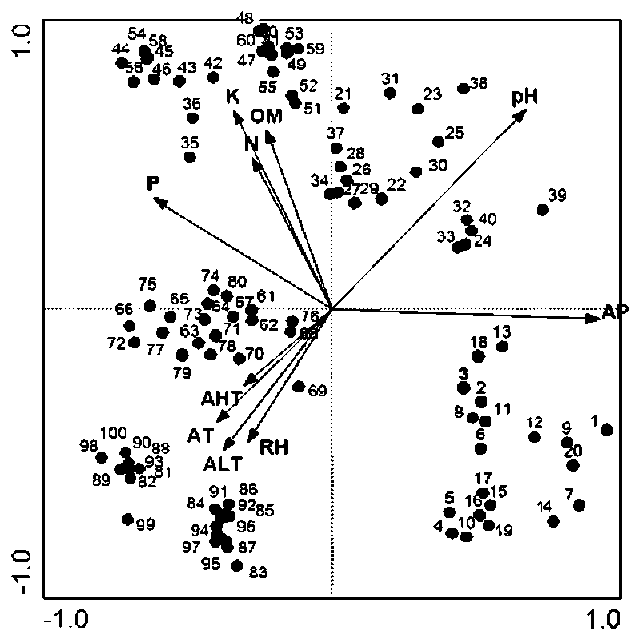
slope and aspect were measured by compass meter. From each quadrat, five soil samples of 30 cm in depth were taken using a small spade. These were thoroughly mixed, then one quarter was collected and taken to a laboratory for chemical analysis. In the laboratory, soil samples were air-dried and soil pH, organic matter, available N, available P and available K were measured as soil variables. These variables were selected because they are most important nutrient elements, particularly in arid and semi-arid areas. A 1:2.5 ratio of soil to distilled water suspension was used to measure pH with a Whatman pH sensor meter. Nitrogen content was estimated using Kjeldahl extraction, and the phosphorus content was measured via the HCLO4-H2SO4 Colorimetric method (Molybdovanadate method). Potassium content was measured using an Atomic Absorption Spectrophotometer and the content of organic matter was measured using the K2Cr2O7 - Capacitance method. Climatic data from the nearest weather stations were used.
MATERIALS AND METHODS
Data analysis
Sampling
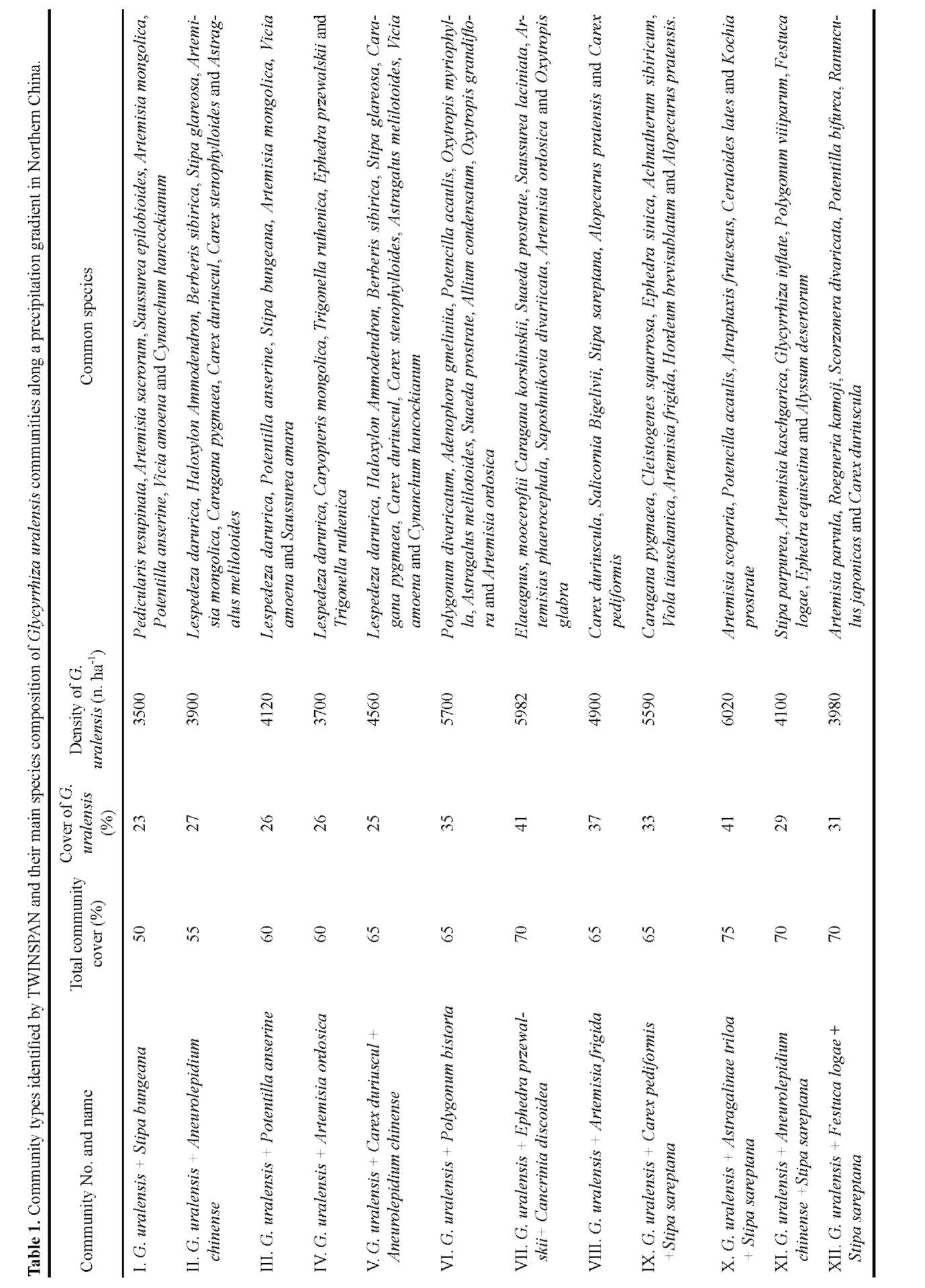
The Importance Value of each species was used as data in community analysis and diversity indices calculation. The importance value was calculated by the formulas (Zhang et al., 2006b):
IVShrubs = (Relative density —Relative cover + Relative height)/3
IVHerbs = (Relative cover + Relative height)/2
The relative density refers to the percentage of one species density over the sum of all species density in a quadrat, relative cover to the percentage of one species cover over the sum of all species cover in a quadrat, and relative height to the percentage of one species mean height over the sum of all species mean height in a quadrat. The species data matrix is consisted of importance values for 191 species in 100 quadrats.
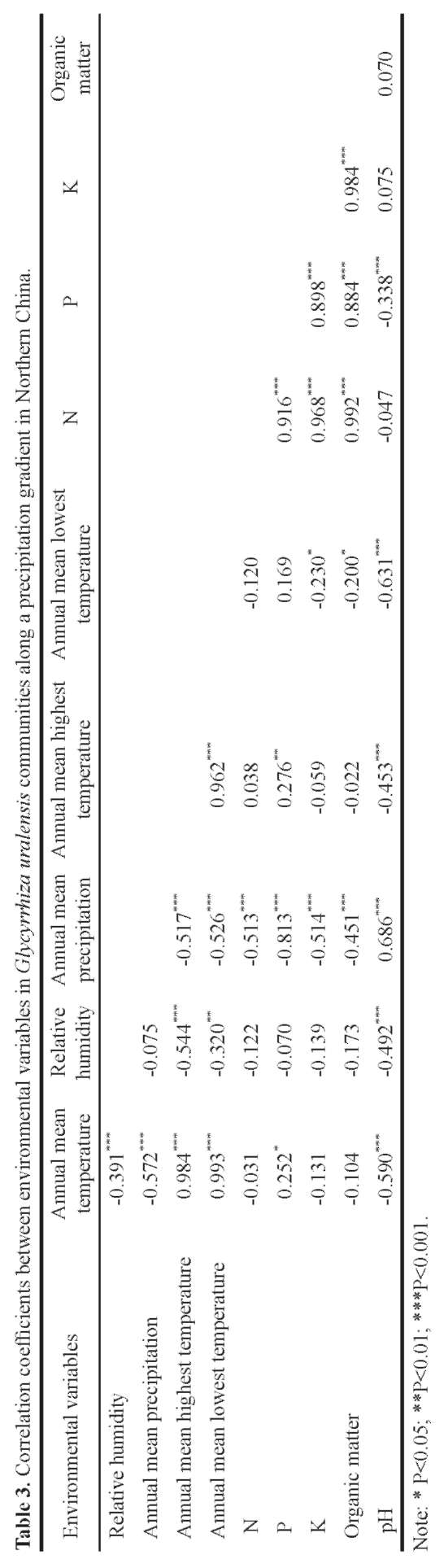
The environmental data matrix consisted of values for ten variables, five soil factors plus five climatic variables, annual mean temperature, relative humidity, annual mean precipitation, annual mean highest temperature and annual mean lowest temperature, in 100 quadrats. Elevation, slope and aspect were not analyzed because their differences were not significant.
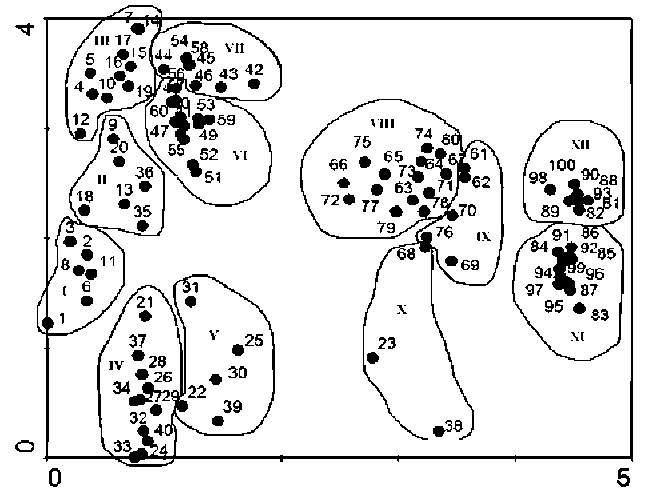
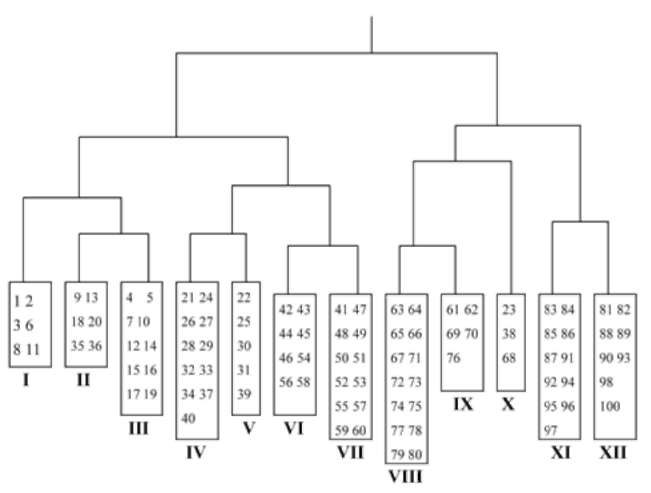
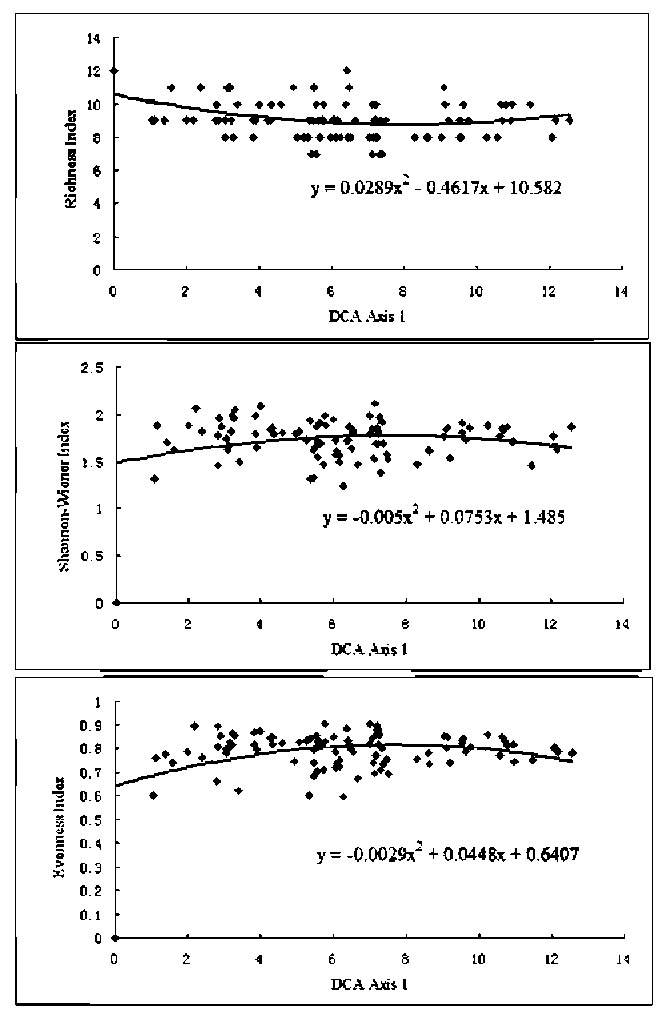
Two-way Indicator Species Analysis (TWINSPAN) (Hill, 1979) was used for classification, Detrended Correspondence Analysis (DCA) and Canonical Correspondence Analysis (CCA) (Ter Braak and Smilauer, 2001) were used for ordination to analyze the variation of communities and their relationships with environmental variables. TWIN-SPAN, DCA and CCA calculations were carried out using TWINSPAN (Hill, 1979) and CANOCO (Ter Braak and Smilauer, 2001) computer programs.
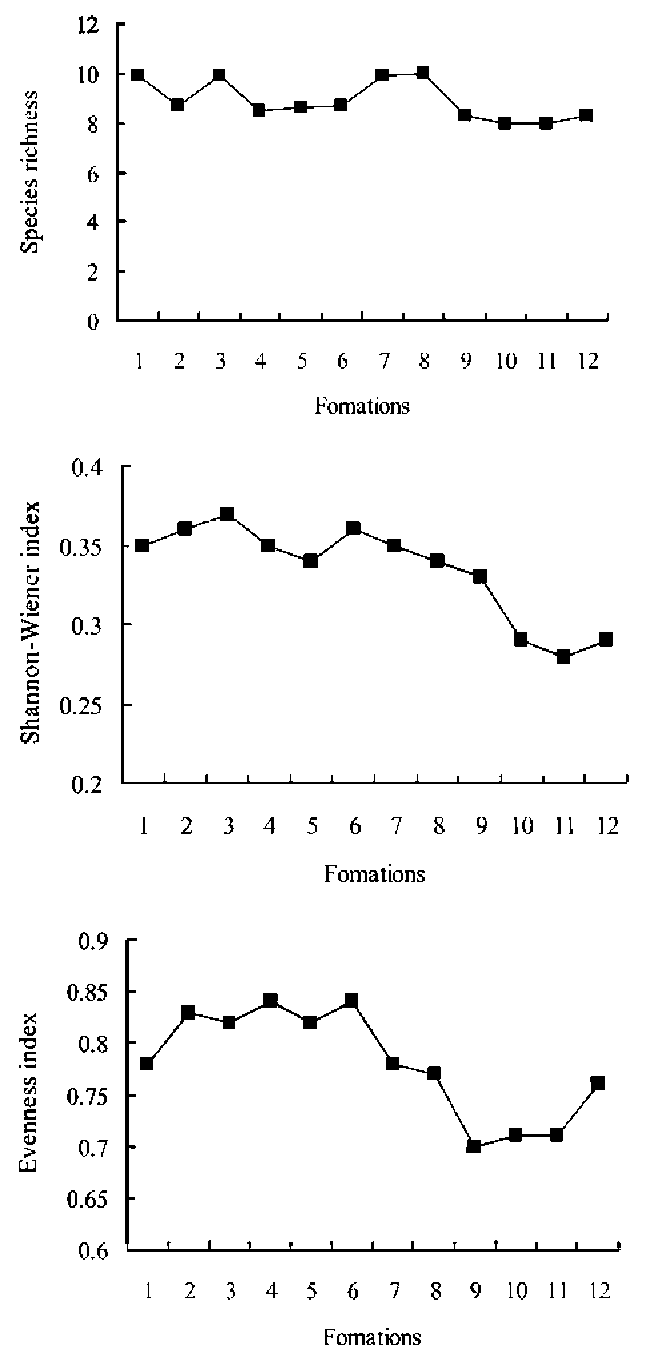
Three separate species diversity indices, for species richness, diversity, and evenness, were used to calculate species diversity in G. uralensis communities. They were:
Species number (as a richness index):
D = S
Based on a general survey of G. uralensis and its communities, a sampling transect across its main distribution area was established from east to west in northern China (Figure 1). Five study sites, Chifeng (in inner Mongolia Autonomous Region), Hengjinqi (in inner Mongolia Autonomous Region), Minqin (in Ganshu Province), Aletai (in Xinjing Autonomous Region) and Kashi (in Xinjing Autonomous Region), were selected as sampling sites (Figure 1). The area of each sampling site was about 40 ha. Twenty quadrats of 5 m x 5 m were established randomly at each site and their respective cover, height, abundance of shrubs and herbs were measured. Plant cover was visually estimated, and heights were measured using a ruler. Altogether, 191 plant species were recorded in 100 quadrats. Elevation, slope and aspect for each quadrat were also measured and recorded. Elevation was measured by GPS,
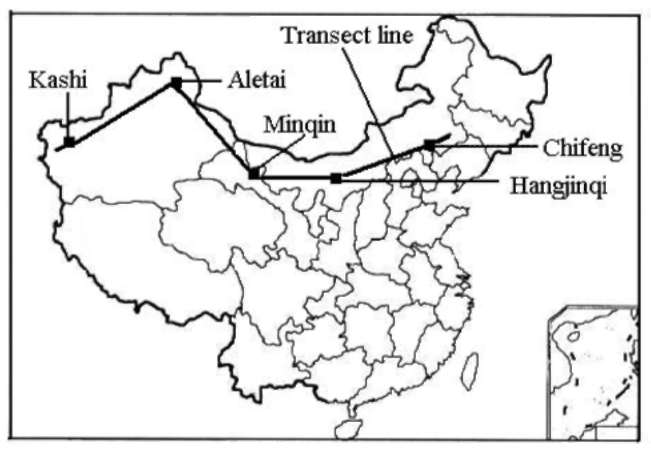
Figure 1. Sampling transect line and sampling sites for Glycyrrhiza uralensis communities along a precipitation gradient in Northern China.