4
Botanical Studies, Vol. 53, 2012
include: Alternaria alternate, Cladosporium herbarum, Leptoxyphium fumagu, Oidium spp. and Phyllosticta cara-ganae (Tomoshevich, 2009). If infected on the roots, the nodules create a cylindrically-shaped infection zone, and the plant will restrict gas and liquid exchange to that area (Allen et al., 1955).
Finally, C. arborescens is also susceptible to several fungi, including Erysiphe palczewskii, a powdery mildew species found in Asia, Europe, Canada and the USA (Va-jna, 2006; Lebeda et al., 2008a,b; Tomoshevich, 2009). Powdery mildew severely affects the leaves and shoots of C. arborescens (Vajna, 2006). Other fungal species com-monly found on C. arborescens leaves include: Micros-phaera trifolii, Uromyces cytisi and Ascochyta borjomi (Lebeda et al., 2008b; Tomoshevich, 2009).
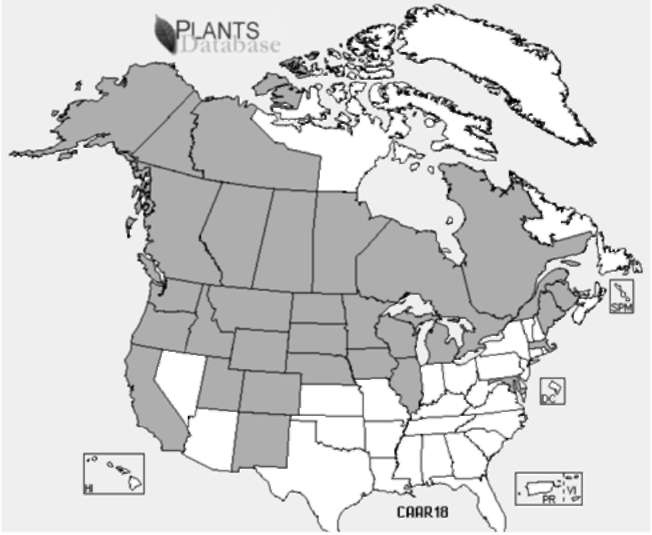
Figure 1. Distribution of the introduced species, C. arborescens, in North America; areas (states, provinces, territories) confirmed to contain C. arborescens are shown in gray, whereas areas from which it is not known to occur are shown in white (USDA NRCS, 2010).
ETHNOBOTANY
Medicinal applications
There is a long history of using Caragana species to treat ailments such as headaches, asthma, cough, nosebleeds and strain-induced fatigue in East Asian folk medicine (Meng et al., 2009). Caragana arborescens has also been used to treat menoxia, fatigue, rheumatoid arth-ritis, asthenia and uterine, cervical and breast cancer. The USDA describes C. arborescens as being used medicinally for breast and uterine cancer and other female anatomy problems (Meng et al., 2009). The two main chemical classes thought to contribute to the medicinal properties of C. arborescens are flavonoids and lectins (Wang et al., 2005; Meng et al., 2009).
to initiate nitrogen fixation at such low temperatures, C. arborescens has a greater northern hardiness limit than most other studied species (Hensley and Carpenter, 1979).
Finally, C. arborescens is a prolific producer of the toxic non-protein amino acid, L-Canavanine (Rosenthal, 2001), which is an allelochemical that provides a barrier to herbivore predation and pathogen uptake. L-Canavanine is structurally similar to L-Arginine and is taken up by in-sects that cannot differentiate between the two amino acids (Rosenthal, 2001).
Flavonoids are phenolic compounds that protect plants from UV radiation and play a part in sexual reproduction (Koes et al., 2005). They are beneficial to humans because they can act as anti-oxidant, anti-inflammatory, anti-cancer, anti-viral and anti-bacterial chemical compounds (Deng et al., 1997; Meng et al., 2009). The flavonoid found in C. arborescens is isoquercetin, which possesses hypoglycemic properties in vitro and has potential as an anti-diabetic agent (Meng et al., 2009).
Enemies
Despite being tolerant of harsh environmental conditions, C. arborescens is susceptible to various herbivores, allelopathic chemicals, pathogens and fungi. Grasshoppers, birds, beetles, moths and deer are known herbivores of C. arborescens (Rosenthal, 2001; Henderson and Chapman, 2006). Two species of aphids, Therioaphis tenera and Aphis craccivora, have been found living on many species of Caragana, and have previously been recorded with C. arborescens (Ripka, 2004). An energy trade-off has been identified for C. arborescens between growth of defensive spines and reproduction, which may initiate the growth of grazing-resistant individuals that are shorter, have smaller leaves, and produce fewer seed pods (Zhang et al., 2006).
Two types of lectins, which function to bind nitrogen-fixing bacteria to their root systems (Barondes, 1981), have been identified in C. arborescens. These lectins can be used as contraception and prophylaxis against sexually transmitted infections (STI) (Meng et al., 2009). C. arbor-escens lectins in vitro selectively eliminated cells infected with the human immunodeficiency virus (HIV) (Meng et al., 2009), suggesting their potential use in the fight against HIV and other sexually transmitted infections. Evidence to date demonstrates the potential of C. arborescens as a medicinal plant; however, more research on its efficacy in treating and eliminating specific conditions is needed.
Under short-term lab conditions, C. arborescens was susceptible to the allelopathic chemicals of the black walnut tree, Julans nigra (Rietveld, 1983). Seed germination of C. arborescens was inhibited by juglone, an allelopathic chemical released by the black walnut tree. However, these results were based on high concentrations of juglone, which may not be attained in nature.
Cultural uses
Caragana arborescens has been globally cultivated for soil stabilization and mine and construction site improvement through nitrogen fixation (Wills, 1982; Meng et al.,
Caragana arborescens is susceptible to various pathogens. Spot-forming pathogens that have been identified