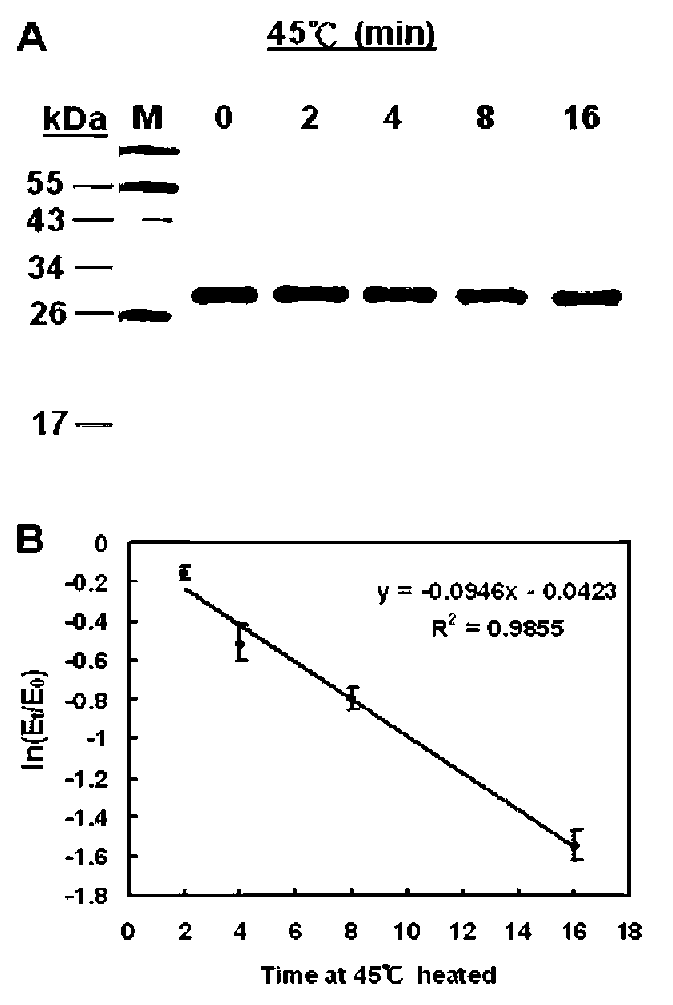
DAI et al. ― Lemon ascorbate peroxidase
11
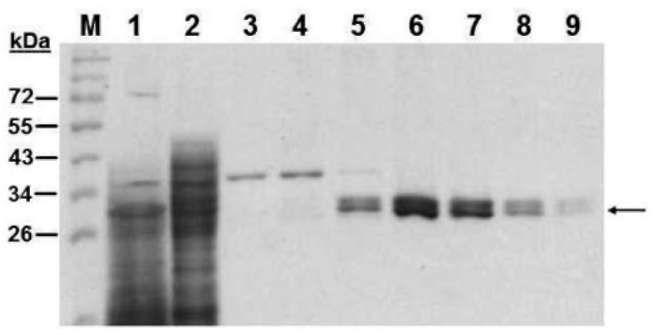
containing 100 mM imidazole) according to the manufacture's instruction (Qiagen). The purified protein was checked by 15% SDS-PAGE. Proteins on the gel were detected by Coomassie Brilliant Blue R-250 staining. Protein concentration was determined using a Bio-Rad Protein Assay Kit (Richmond, CA) using bovine serum albumin as a standard.
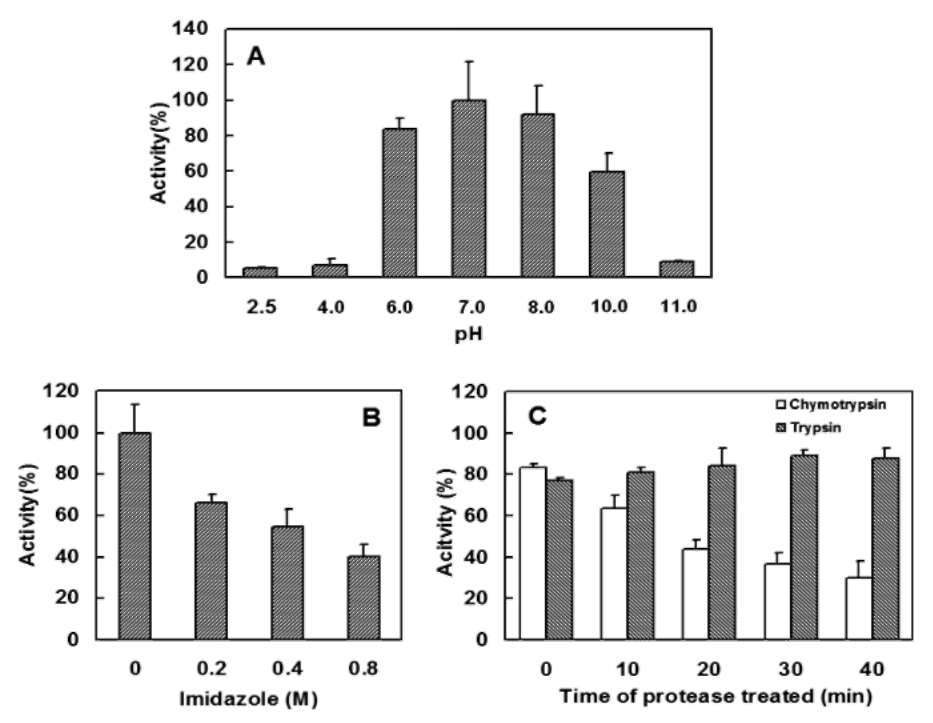
added to the enzyme sample (0.2 [g/2 [L enzyme in 3% PBS containing 5% glycerol per reaction) to the final levels (each final volume is 20 iL) of 0.2, 0.4, or 0.8 M and incubated at 37°C for 1 h. (4) Proteolytic susceptibility. The enzyme (1.0 [g/10 [L in 1.5 % PBS containing 2.5% glycerol, additional 10 mM CaCl2 for chymotrypsin) was incubated with one-tenth of trypsin or chymotrypsin (w/w) at pH 8.0, 37°C for a period of 5, 10, 20 or 40 min. Ali-quots (0.2 [g/2 [L) were removed at various time intervals for analysis. After each treatment, the residue Apx activity was tested as described above.
Electrospray ionization quadrupole-time-of-flight (ESI Q-TOF) molecular mass analysis
The purified recombinant ClApx (0.1 mg/mL) was dissolved in 3% PBS containing 0.05 mM imidazole and 0.5% glycerol. The sample (5 [L) was used for molecular mass determination using an ESI Q-TOF mass spectrometer (Micromass, Manchester, England).
Apx activity assay and kinetic studies
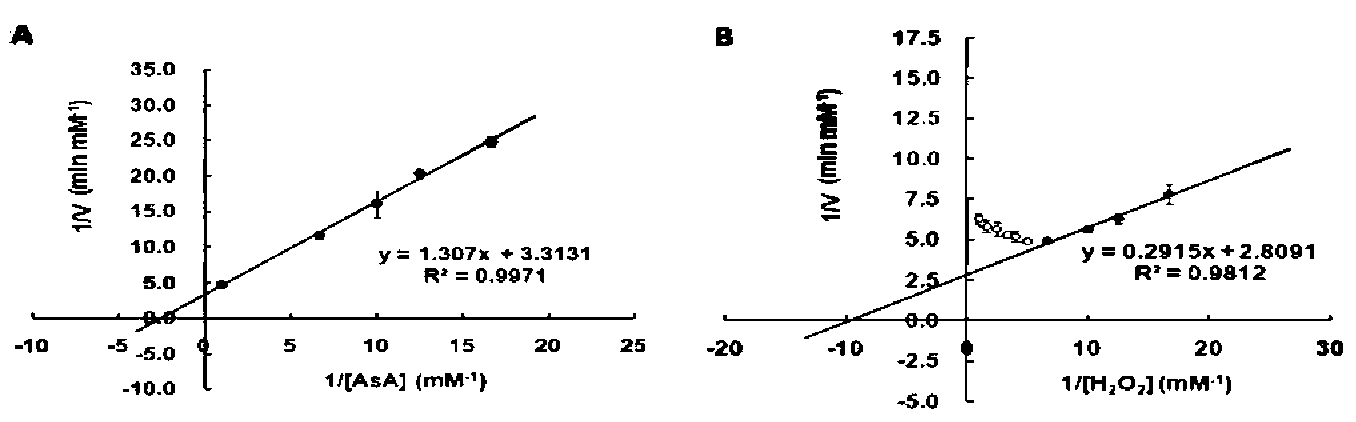
Apx activity was determined by measuring AsA oxidation (Nakano and Asada, 1981). The reaction mixture (100 [L) contained 20 mM potassium phosphate (pH 7.4), 1 mM H2O2, 1 mM AsA and 0.2 [g ClApx. The reaction started upon the addition of ClApx. The reaction was followed by a decrease in A290 due to AsA oxidation.
ClApx (0.2 [g) kinetic properties were determined by varying the concentrations of AsA (0.06 to 1.0 mM) with the fixed amount of 1 mM H2O2 or varying concentrations of H2O2 (0.06 to 1.0 mM) with the fixed amount of 1 mM AsA. The change in absorbance at 290 nm was recorded between 10 sec and 20 sec. The molar absorption coefficient of AsA at 290 nm was 2.8 mM-1 cm-1. The Km, Vmax and kcat were calculated from Lineweaver-Burk plots.
Biochemical characterization
The stability of ClApx under various conditions was studied by assaying its peroxidase activity. Aliquots of the ClApx sample were tested for: (1) Thermal effect. Enzyme sample (0.2 [g/2 [L enzyme in 3% PBS containing 5% glycerol per reaction) was heated to 45°C for 2, 4, 8 or 16 min. (2) pH effect. Enzyme sample (0.2 [g/2 [L enzyme in 3% PBS containing 5% glycerol per reaction) was adjusted to the desired pH by adding a volume of buffer with
different pHs: 0.2 M citrate buffer (pH 2.5, 4.0), 0.2 M
potassium phosphate buffer (pH 6.0, 7.0 or 8.0) or 0.2 M CAPS buffer (pH 10.4, or 11.0). Each sample was incubated at 37°C for 1 h. (3) Imidazole effect. During protein purification, the ClApx enzyme was eluted with imidazole and its effect on activity was examined. Imidazole was
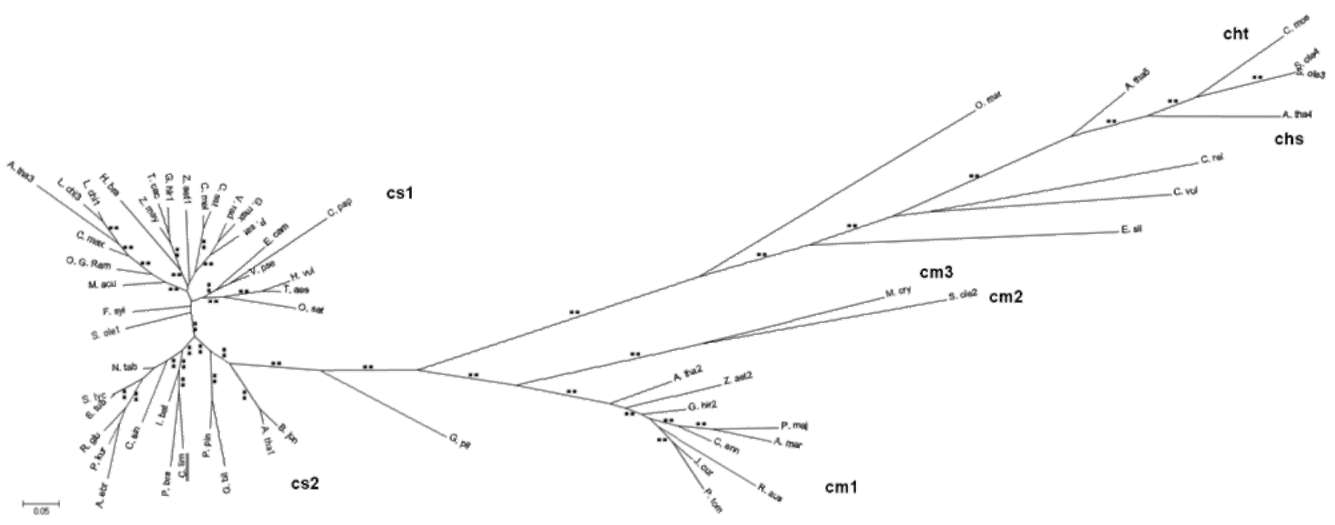
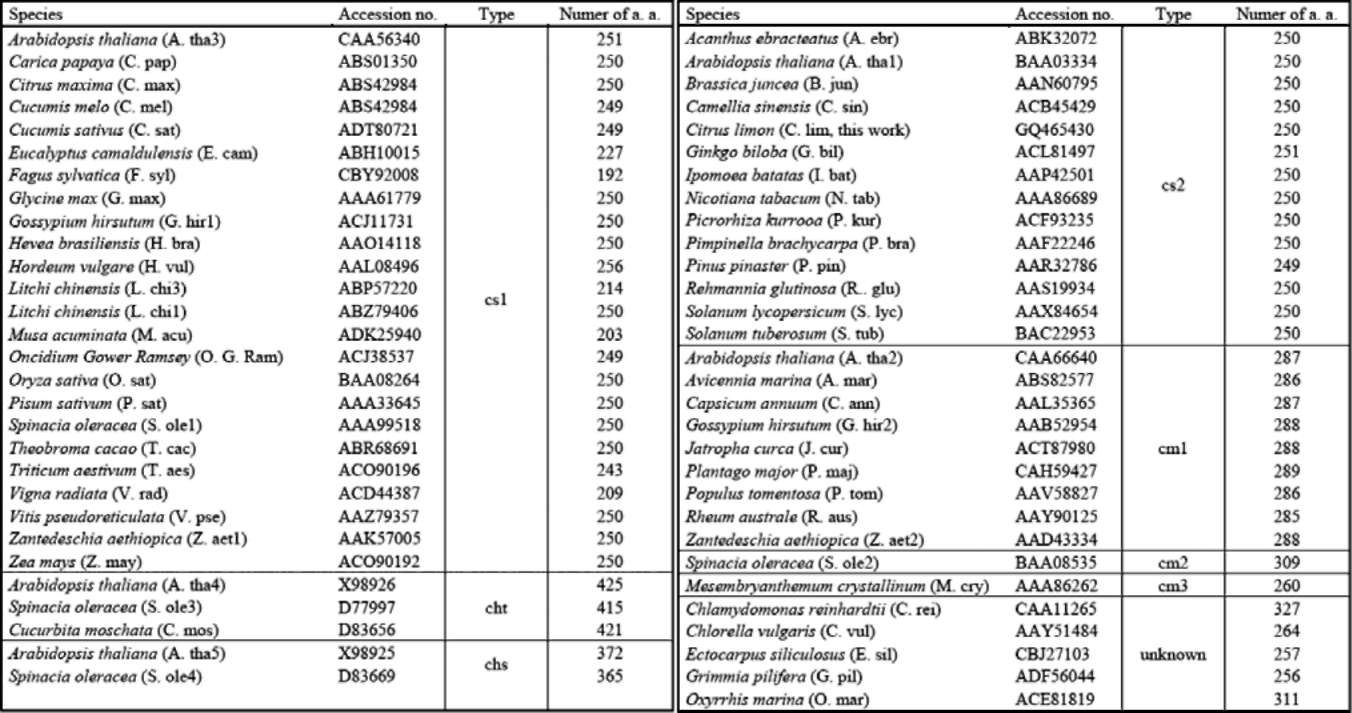
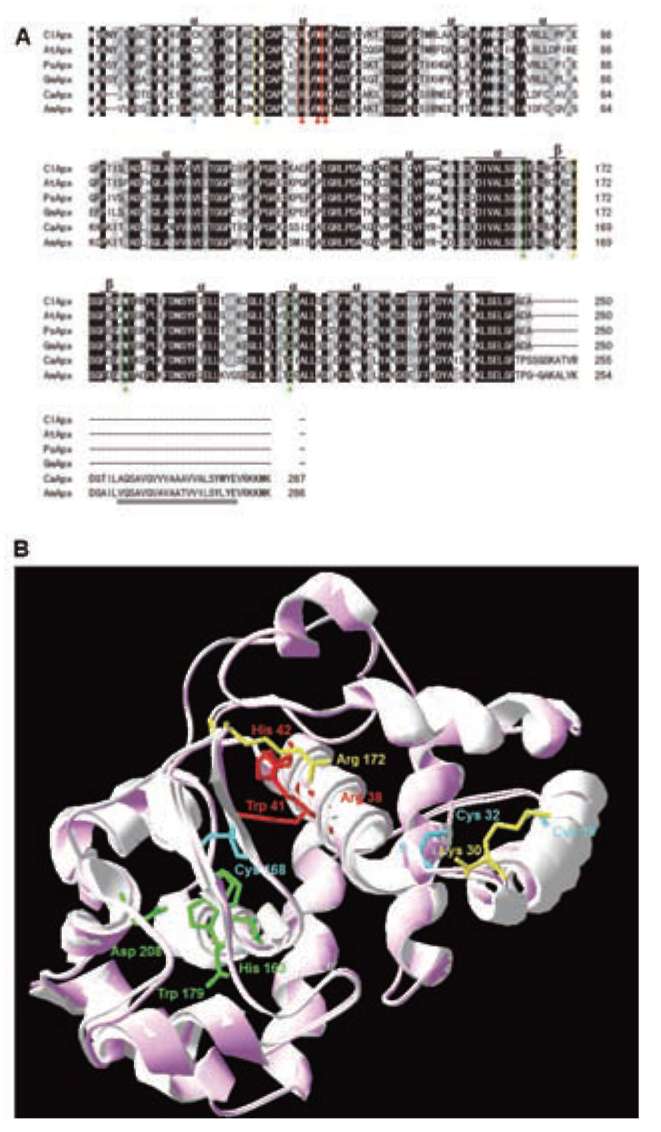
Figure 1. Alignment of the ClApx amino acid sequences with other organism's Apxes and 3-D structural model. (A) Sequence alignment: ClApx (this study), AtApx (Arabidopsis thaliana), PsApx (Pisum sativumf), GmApx (Glycine max), CaApx (Capsicum annuum) and AmApx (Avicennia marina). Conservative replacements are shaded gray. Protein secondary structure was predicted by the SWISS-MODEL program and predicted a helices and p strands are indicated; (B) A 3-D structural model of ClApx was modeled based on the known X-ray structure of PsApx (Pisum sativum) via the SWISS-MODEL program and was superimposed to obtain a better structure via the SPDBV_4 program. Superimposition of ClApx (pink) and GmApx (Glycine max) (white) was shown using protein solid ribbons. Blue stars denote the 3 Cys residues in the ClApx protein. The yellow triangles indicate the putative AsA binding sites. The red triangles indicate the putative active sites. The green triangles denote the conserved residues of the putative proximal hydrogen bonded triad. Double underline denote the C-terminal membrane-spanning segment belonging to cm1 type.