20
Botanical Studies, Vol. 53, 2012
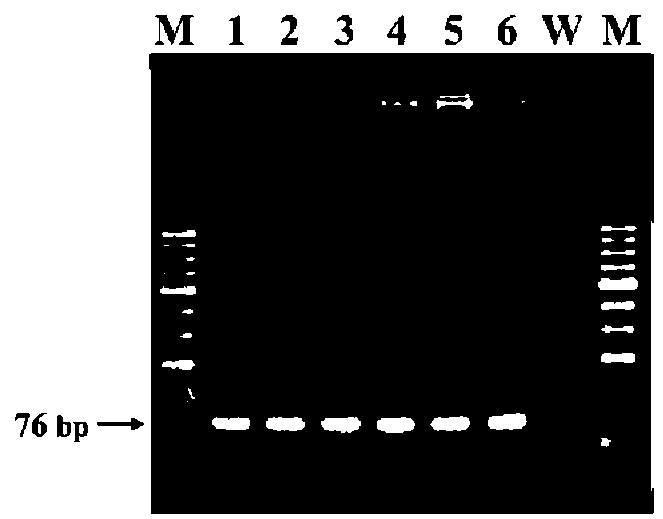
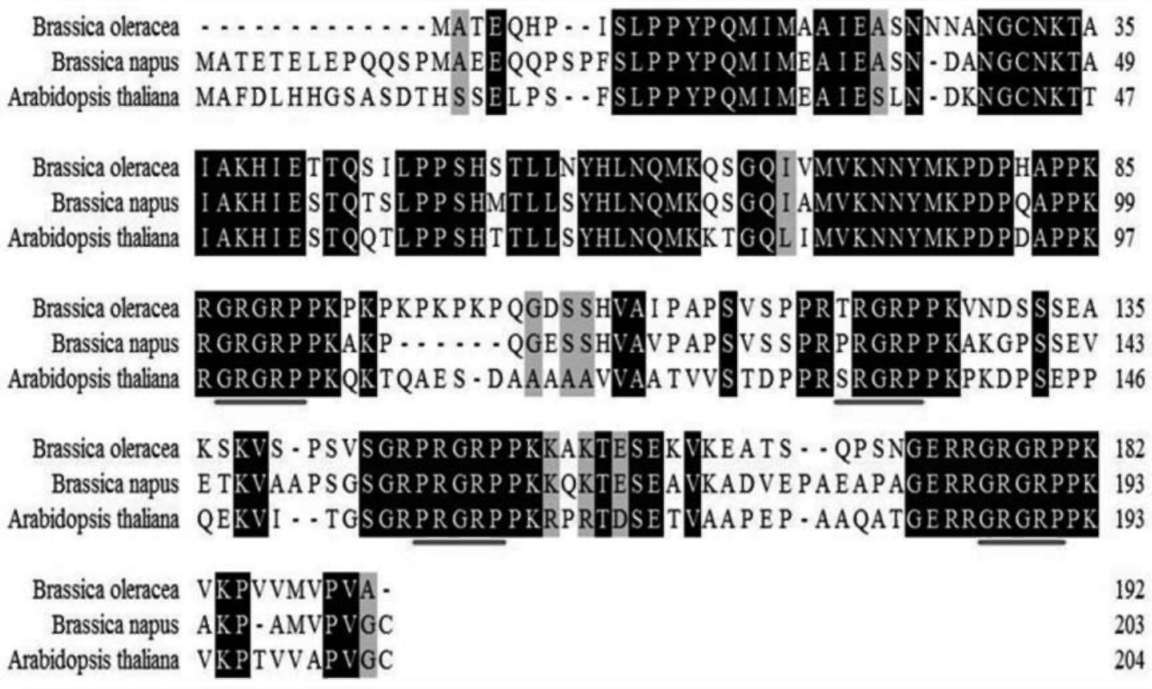
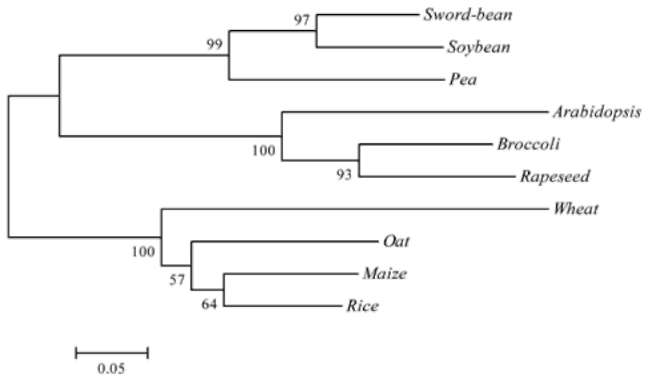
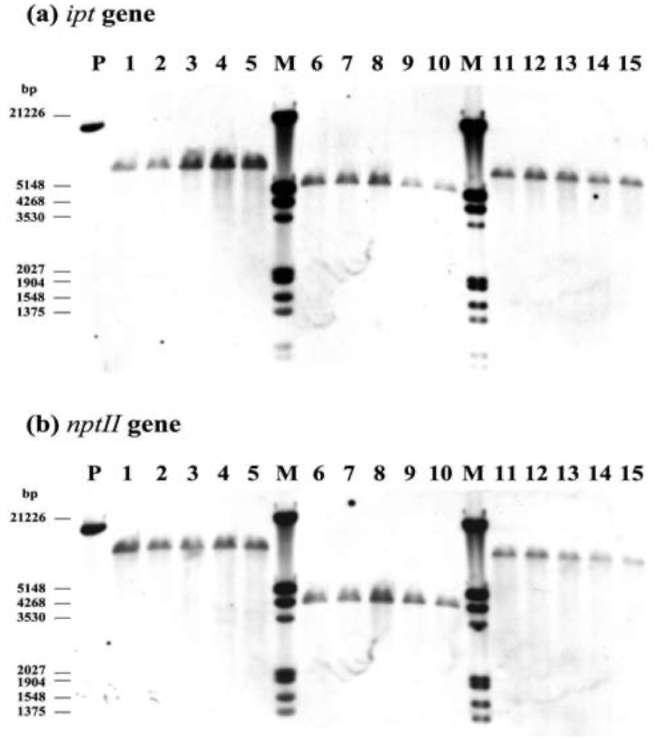
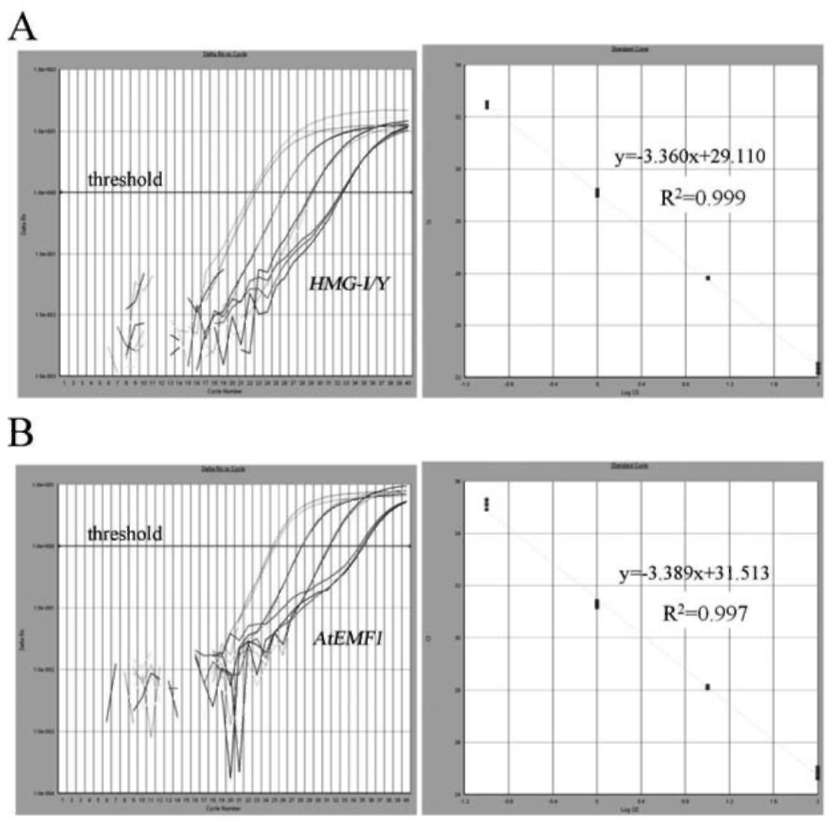
lution of a known sample, while the relative quantification relies on comparing the amplified signal with the control gene. Estimating this requires exploring the endogenous genes and their reference gene potentials. Ding et al. (2004) propose that a reliable reference gene should meet three requirements: be species specific, have low copy number, and have low heterogeneity among the cultivars. They provided reference plant gene examples for maize, soybean, rape-seed and rice. The most intensive research on endogenous reference genes for qRT-PCR studies in Brassica have been with rapeseed (Brassica napus) (Masek et al., 2000; Weng et al., 2004; Weng et al., 2005; Wu et al., 2010). Wu et al. (2010) indicated that there were five endogenous reference genes in B. napus: acetyl-CoA carboxylase gene (BnAC-Cg8), phosphoenolpyruvate carboxylase (PEP), oleoxl hydrolase gene (Fat A), high-mobility-group protein I/Y gene (HMG-I/Y) and cruciferin A gene (Cru A), The presence of these genes in other Brassica species is currently contested. For example, Weng et al., 2005, pointed out that HMG-I/Y is a single copy in rapeseed and that its amplification was not observed in other Brassica genus or species, such as broccoli, stem mustard, cauliflower, Chinese cabbage, cab國 bage, sprouts, etc. This gene was also absent in B. oleracea according to Southern blotting analysis of the HMG-I/Y gene. Wu et al. (2010), however, recently located HMG-I/Y in B. napus, B. nigra and B. oleracea. In this study, we demonstrate the presence of HMG-I/Y as single copy in broccoli (B. oleracea var. italica) and its utility for copy number determination in transgene broccoli transformation.
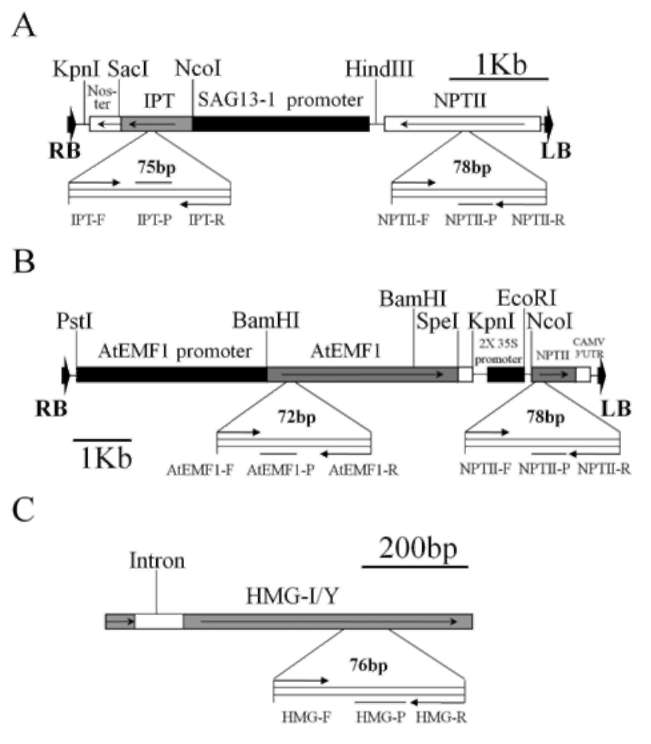
Figure 1. Maps of transgene constructs and sites of primer sets designed for qRT-PCR, (A) ipt&NPTII; (B) AtEMF1&NPTI! and (C) endogenous Brassica oleracea var. italica HMG-I/Y.
for copy number determination to validate the established methodology.
MATERIALS AND METHODS
Plant DNA extraction, total RNA isolation and cDNA synthesis
Plant materials and transgene constructs
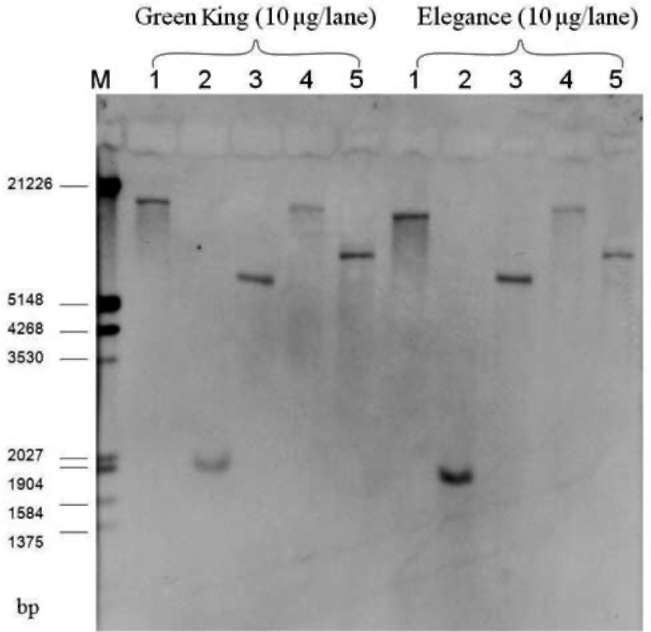
The plant genomic DNA samples used for quantitative PCR detection were extracted and purified using a DNA extraction kit: Plant Genomic DNA Extraction Miniprep System (VIOGENE, Taipei, Taiwan, R.O.C.). The plant genomic DNA samples used for the Southern blot analysis and chromosome walking were extracted and purified from fresh leaves according to the CTAB method (Gawel et al., 1991). Genomic DNA was quantified spectrophotometri-cally using a DU® 640B DNA/Protein calculator (Beckman Company) and analyzed by 1% agarose gel electrophoresis in lxTBE with ethidium bromide staining.
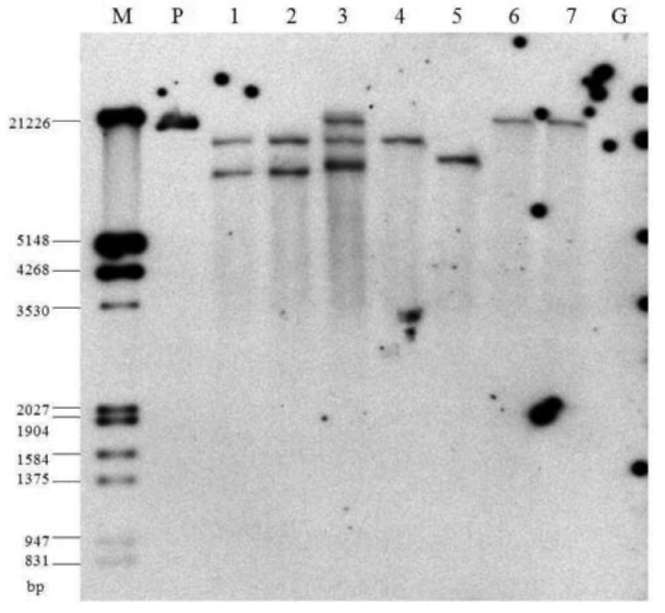
Broccoli (B. oleracea var. italica) cultivars Green King and Elegance seeds were obtained from the Know-You Seed Company (Kaoshiung, Taiwan). We used constructs of transgene ipt and AtEMF1 as indicated in Figure 1A and 1B. We also used transgenic ipt T5 lines derived from self-progeny of selected T4 individuals from our previous study (Chen et al., 2001). Three T4 lines were used first to check the stability and purity among selfed or sib-mated (due to the occasional lack of pollen) progeny. For each line, five sib or self-pollinated progeny were randomly selected for transgene zygosity examination via qRT-PCR. Among those, Lines 101 and 103 derived from the same T。 trans-formant and Line 102 derived from another independent transformant. At least ten individuals of each T5 from the self-pollinated progeny of each line were further confirmed for the copy number. The agronomic performance of the developed inbred lines (T5) has been reported elsewhere (Chan et al., 2009). The later transgene construct for polycomb gene study (Figure 1C) was kindly supplied by Professor Zinmay Renee Sung of the Department of Plant and Microbial Biology, University of California, Berkeley, US). The AtEMF1 transgenic lines were obtained following the method of Chen et al. (2001) using Agrobacterium-mediated transformation. We used twenty-four T0 plants
Total RNA was extracted from broccoli leaves using an RNeasy Plant Mini Kit (Qiagen), according to the manufacturer's instructions. Total RNA were reverse transcribed with Reflection™ Kit (ACTIVE MOTIF) and oligo (dT) following the enzyme manufacturer's instructions.
Cloning and identifying the broccoli HMG-I/Y gene
Primer pairs were designed to amplify the partial HMG-I/Y cDNA of broccoli through comparisons of NCBI blastn
(http://www.ncbi.nlm.nih.gov/BLAST/) alignments on HMG-I/Y cDNAs of Arabidopsis thali-ana (GenBank accession number CAA71797) and