64
Botanical Studies, Vol. 53, 2012
assays used in this study measured the oxidative products at the early and final stages of oxidation. Antioxidants have different functional properties, for example quer-cetin, rutin, and catechin can scavenge reactive oxygen species (Liu et al., 2008); p-coumaric acids, on the other hand, inhibit the generation of free radicals and chain-breaking activity (Laranjinha et al., 1995) and metal chela-tion (Van-Acker et al., 1998). These compounds, as well as flavonoids and other organic acids, are highly effective electron donors. However, the components responsible for the antioxidative activities of the wetland medicinal plants are still unclear. Further work must be performed to isolate and identify these components.
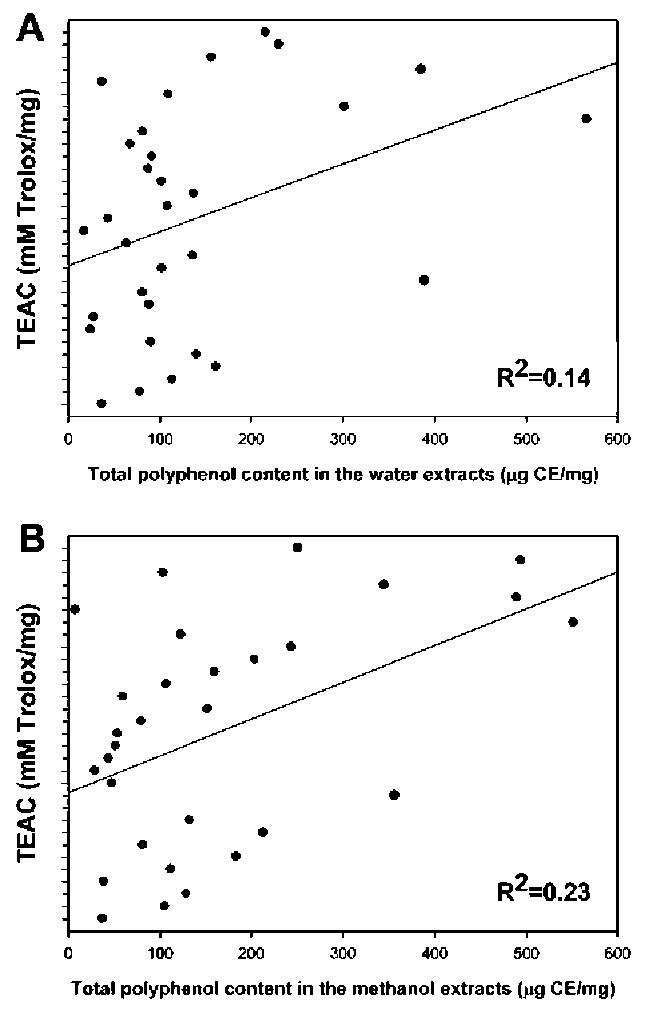
In conclusion, the results from these in vitro experiments, including ABTS radical monocation scavenging (Table 2), DPPH radical scavenging (Table 3), reducing power method (Table 4), total polyphenol content, total flavonoid content and total flavonol content (Table 5 and 6), demonstrated that phytochemicals in wetland medicinal plants might have significant effects on antioxidant activities. However, the quantity of polyphenols and flavonoids found in the wetland medicinal plant extracts were not directly related to their antioxidant activities. The additive roles of phytochemicals might contribute significantly to the potent antioxidant activity. Hence, some wetland medicinal plants could be used as an easy accessible source of natural antioxidants in pharmaceutical and medical industries. For this reason, further work should be performed to isolate and identify the antioxidative components of wetland medicinal plants.
Acknowledgements. This study was supported by a
grant CCMP-97-RD-001 from the Committee on Chinese
Medicine and Pharmacy, Department of Health, Executive
Yuan, Taiwan. And China Medical University (CMU)
(CMU99-S-29, CCM-P99-RD-042, and CMU99-COL-
10), Taiwan Department of Heath Clinical Trial and Research
Center of Excellence (DOH100-TD-B-111-004)
and the Cancer Research Center of Excellence (DOH100-
TD-C-111-005).
Figure 1. Correlation coefficients (R2) of TEAC and total polyphenol contents in the water (A) and methanol (B) extracts of the wetland medicinal plants.
Typha orientalis is a commonly used Chinese herbal drug which has been shown to possess blood circulation stimulating, hypertension relieving and nerve soothing effects. There are no prior reports on the antioxidant activity of this plant.
Phenolic compounds, such as flavonoids, phenolic acid and tannins, possess anti-inflammatory, anti-carcinogenic, anti-atherosclerotic, and other properties that may be related to their antioxidant activities (Chung et al., 1998; Wong et al., 2006). Flavonoids and flavonols are two poly-phenolic compounds that play an important role in stabilizing lipid oxidation and are associated with antioxidant activity (Yen et al., 1993). Phenolic compounds may contribute directly to antioxidative action (Duh et al., 1999). Polyphenolic compounds may have an inhibitory effect on mutagenesis and carcinogenesis in humans when as much as 1.0 g is ingested daily from a diet rich in fruits and vegetables (Tanaka et al., 1998). The antioxidative activities observed can be attributed to both the different mechanisms exerted by different phenolic compounds and to the synergistic effects of different compounds. The antioxidant
LITERATURE CITED
Ali, B.H., G. Blunden, M.O. Tanira, and A. Nemmar. 2008. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 46: 409-420.
Arnous, A., D.P. Makris, and P. Kefalas. 2001. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 49: 5736-5742.
Awika, R., P. Wu, J.M. Cisneros-Zevallos, L.W. Awika, XI. Rooney, R.L. Wu, and L. Cisneros-Zevallos. 2003. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products, J. Agric. Food Chem. 51: 6657-6662.