WANG and HSIEH ― SIJRL1 expression delays bacterial wilt
77
|
Plasmid constructs and transformation
|
||
|
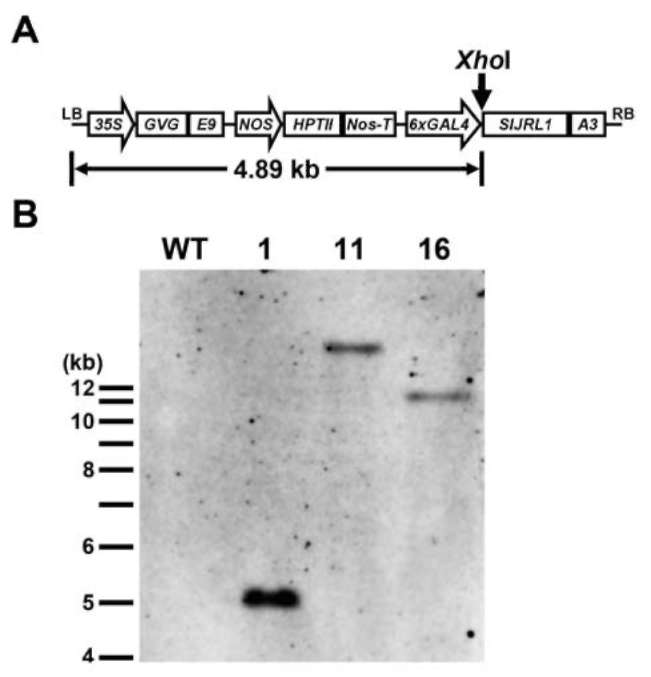
The cDNA fragment of SlURL1 was amplified with the primers 5'-TCTAGAATGAAGATGATGGT GGAAAATATTG-3' and 5'-ACATTGGGACGACCGG TAAGATCT-3' by RT-PCR with the template generated from the total RNA of tomato roots. The resulting cDNA fragment was cloned into yT&A vector (Yeastern Biotech. Co.) and named yT&A/SlJRL1 for sequence verification. SlJRL1 was then excised from yT&A/SlJRL1 as aXbaI fragment and sub-cloned into thepTA7002 vector (Aoyama and Chua, 1997) with a DEX-inducible promoter. The pTA7002/SlURL1 was then transformed into CL5915 via Agrobacterium-mediated transformation (Chan et al., 2005).
|
||
|
Infection with R. solanacearum
|
||
|
Transgenic seeds of the T1 generation were screened on MS medium supplemented with 20 [ig/ml hygromycin for two weeks, and wild-type seeds were germinated on MS medium alone. The hygromycin-resistant T1 tomato plants and wild-type plants were transferred to 2-inch-diameter plastic pots containing sand, soil, rice husks, and compost (1:3:1:1) and incubated at 28°C under 16-h light/8-h dark for two weeks. The preparation of the inoculum R. solan-acearum strain Pss4 was described previously (Lin et al., 2008). Briefly, bacteria were grown on 523 medium (0.3 g/l MgSO4'7H2〇,2.0 g/l K2HPO4, 4.0 g/l yeast extract, 8.0 g/l casein hydrolysate, 10.0 g/l sucrose, 15g/l agar) supplemented with 50 mg/l TTC (2,3,5-triphenyltertrazo-lium chloride) at 28°C for 24 h, harvested and suspended in sterile water to adjust the concentration to 108 colony
|
||
|
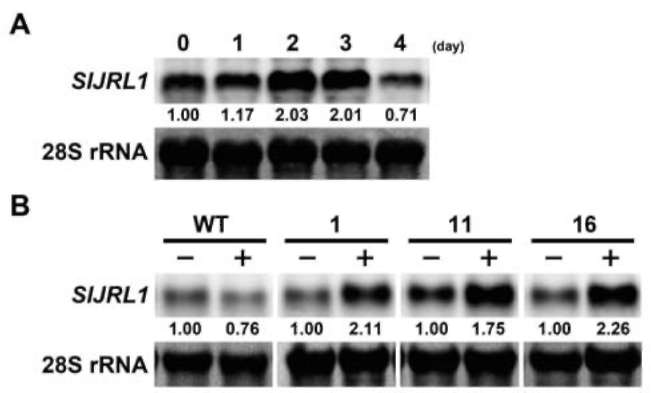
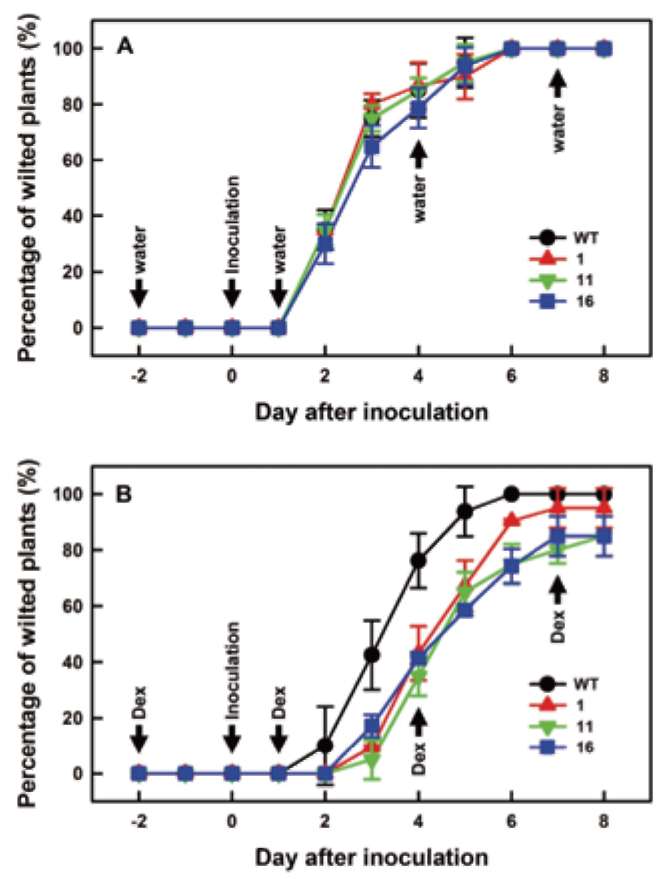
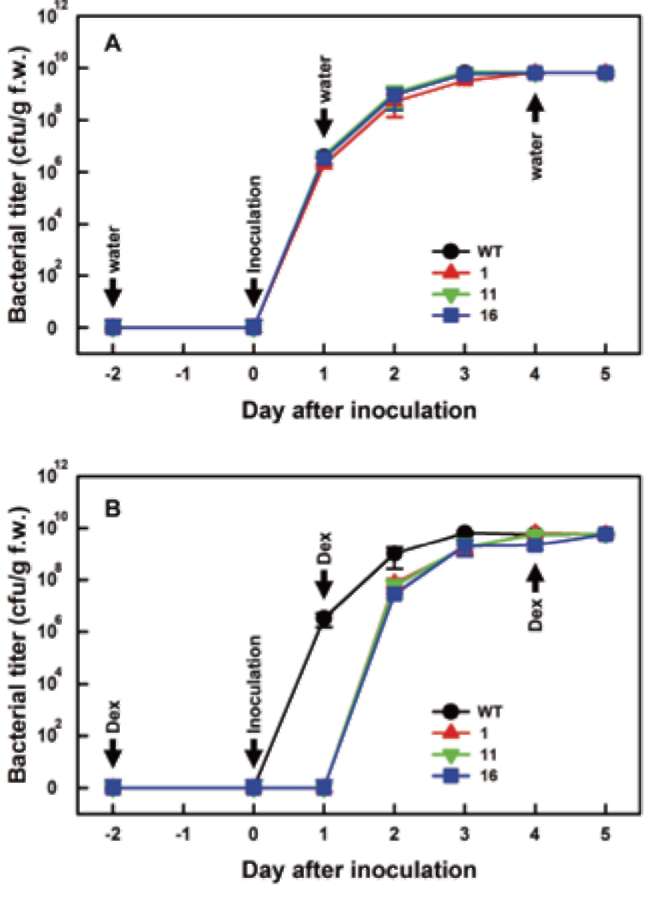
forming units/ml. Roots of four-week-old tomato plants were damaged with a knife, and bacteria inoculum was poured into pots. Inoculated tomato plants were incubated at 28°C to develop wilt symptoms. For the induction of transgenic SlJRL1 expression, 5 μM DEX was added to each pot at the indicated time. As a control, 75 ml water was added to pots. Two biological experiments were performed, each experiment with three replicates, and each replicate involved ten plants.
|
||
|
Analysis of bacterial growth
To detect the propagation of bacteria, tissue 2 cm below the shoot apex of infected tomato plants was collected at different times and ground in sterile water. The extracts were then serially diluted with sterile water, and the ti-ter of bacteria was analyzed on SM1 medium (100 mg/l polymyxin B sulfate, 20 mg/l tyrothricin, 5 mg/l chloram-phenicol, 5 mg/l cycloheximide, 50 mg/l TTC, 5 mg/l crystal violet) at 28°C (Lin et al., 2008). Five plants were collected at each time, along with three bacterial growth replicates per plant.
|
||
|
results
|
||
|
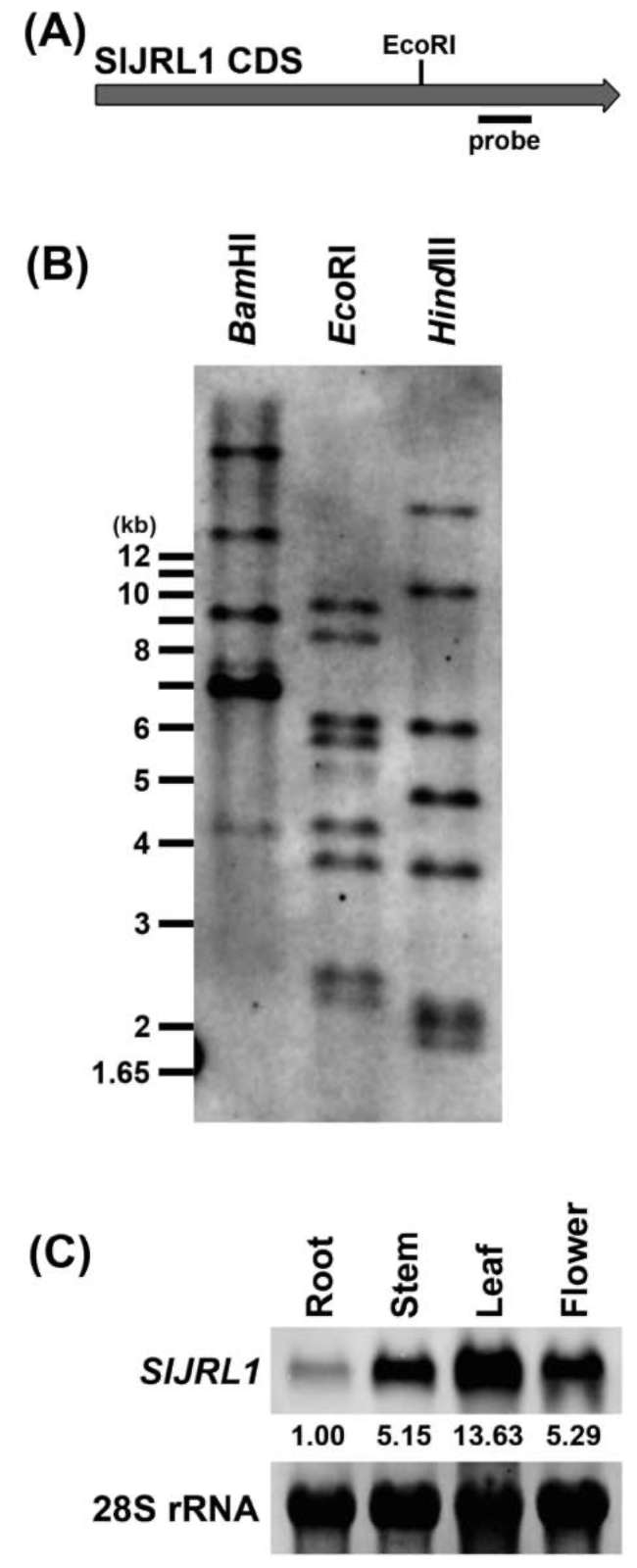
Isolation of tomato JAR1-Iike gene, SIJRL1, from heat-tolerant tomato CL5915
|
||
|
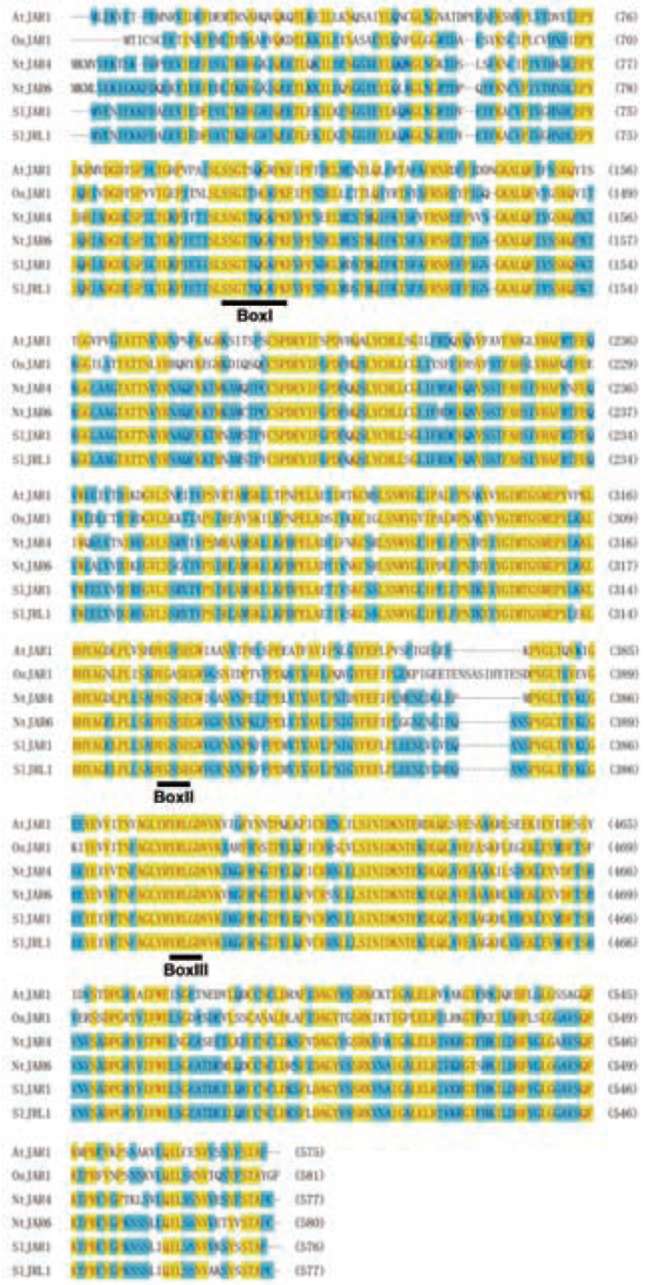
Arabidopsis JAR1/FIN219 is a JA-conjugating enzyme responsible for producing JA-Ile (Staswick et al., 2002) and plays vital roles in far-red light and JA signaling integration (Hsieh et al., 2000; Chen et al., 2007). To obtain tomato homologs of JAR1/FIN219, we searched the to-
|
||
|
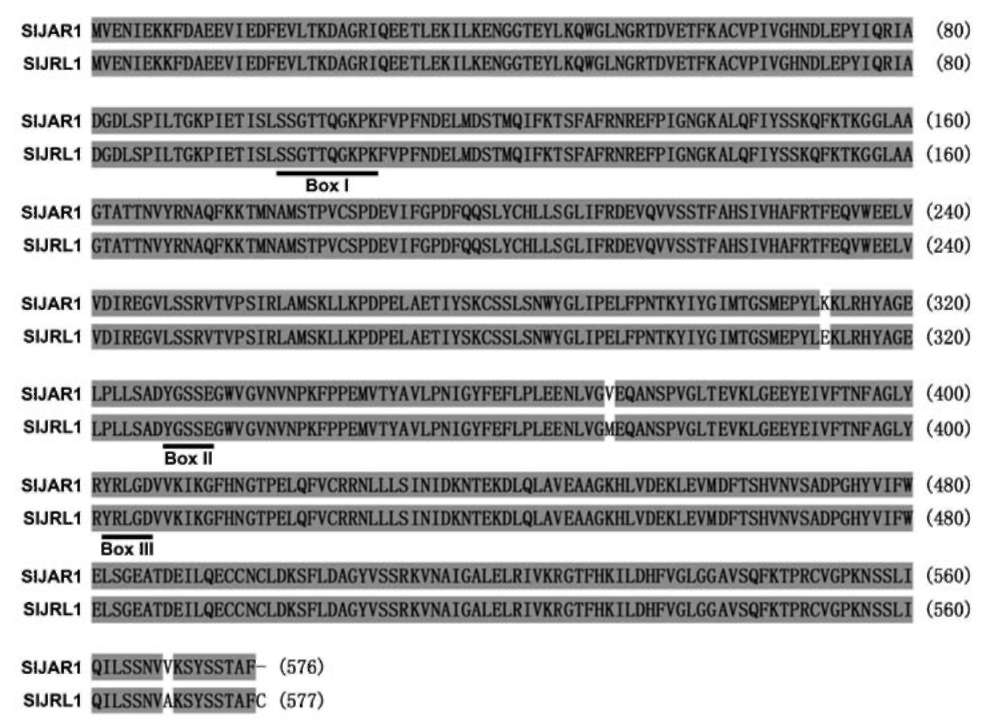
Figure 1. Alignment of Solanum lycopersicum jasmonate-resistant 1 (SlJAR1) and SIJAR1-like 1 (SlJRL1) amino acid residues. The solid lines indicate adenylate-forming domains, boxI, boxII, and boxIII. Different amino acid residues between S1JAR1 and SlJRL1 are not shaded.
|
||