CHEN et al. ― Growth and ammonia uptake by microalgae
127
Table 2. Dry weight (pg) per cell ot marine microalgae at various temperatures. Data represent the mean 士 the standard deviation. Different letters indicate significant differences in a specific species of microalgae among different temperatures (p<0.05).
|
|
|
|
Temperature (°C)
|
|
|
||
|
Species
|
15
|
20
|
25
(Dry weight (pg) cell-1)
|
30
|
35
|
||
|
Nannochloropsis oculata
|
11.9±0.91a
|
9.8±0.42b
|
10.3±0.51b
|
13.0±0.84a
|
|
||
|
Isochrysis aff. galbana
|
46.2+2.0
|
46.7+4.0
|
40.4+1.4
|
51.8+3.0
|
|
||
|
Chaetoceros muelleri
|
35.4±1.6b
|
41.7+4.2b
|
39.7+2.2b
|
39.4+3.4b
|
81.4+3.7a
|
||
|
Tetraselmis chui
|
441±14b
|
424+8.7b
|
369+29bc
|
539+15a
|
|
||
|
Dry weight. This assay was based on a method described by D'Souza and Kelly (2000). The dry weight of the microalgae was determined by filtering 100 mL of microalgae onto precombusted glass-fiber filters (Whatman GF/C for T. chui, I. aff. galbana, and C. muelleri; GF/ F for N. oculata, 47 mm in diameter) and washing with 0.5 M ammonia formate to remove residual salts from the culture. The filters were then dried at 60°C until a constant weight was reached.
|
||
|
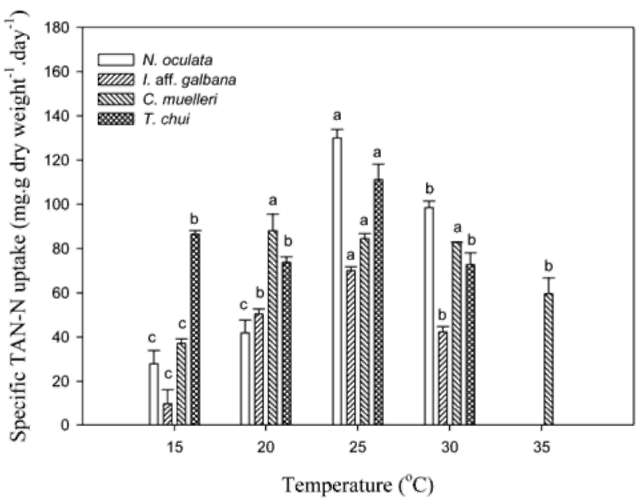
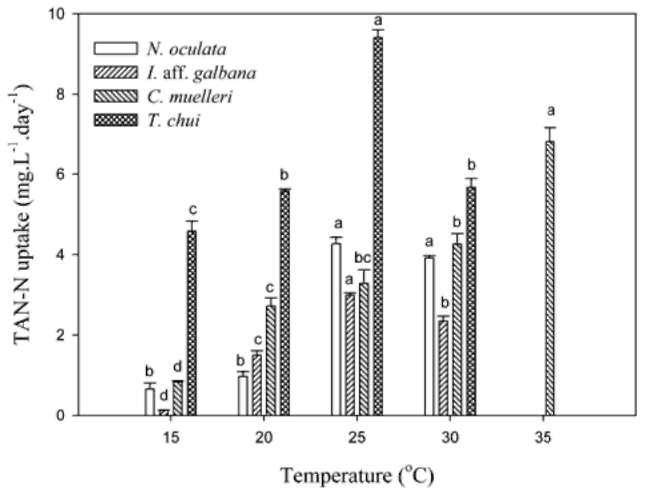
TAN remaining and specific uptake. Twenty mil-liliters of microalgal medium was sampled and centrifuged at 14,000 xg for 15 min at 4°C. After centrifugation, the supernatant was removed and used to determine the TAN concentration. TAN determination was according to the phenol hypochlorite method in APHA et al. (1995). Specific TAN uptake (qt) was calculated by using the equation below:
|
||
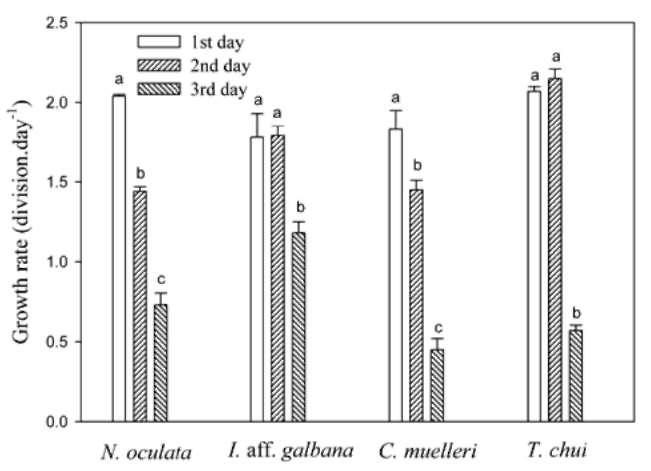
Figure 1. Growth rates of four microalgae species on the first, second, and third days of culture at 25°C. Each bar represents the mean value with the standard deviation. Different letters indicate a significant difference in a specific species of microalgae among different days culture (p < 0.05).
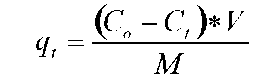
|
where Co and Ct (mg-L-1) represent the TAN concentration initial and at t time in the medium. V(L) is the volume of the medium, M(g) is the biomass (g).
|
||
|
Data analysis
|
||
|
The mean values of growth rates, and of TAN removal at studied conditions were compared by variance analysis (ANOVA) using SigmaStat statistical software from SPSS (SPSS, Chicago, IL, USA), ver. 10. Duncan's test for pair-wise comparisons was used at the 5% significance level.
|
||
|
RESULTS
|
||
|
Dry weights per cell of marine microalgae
|
||
|
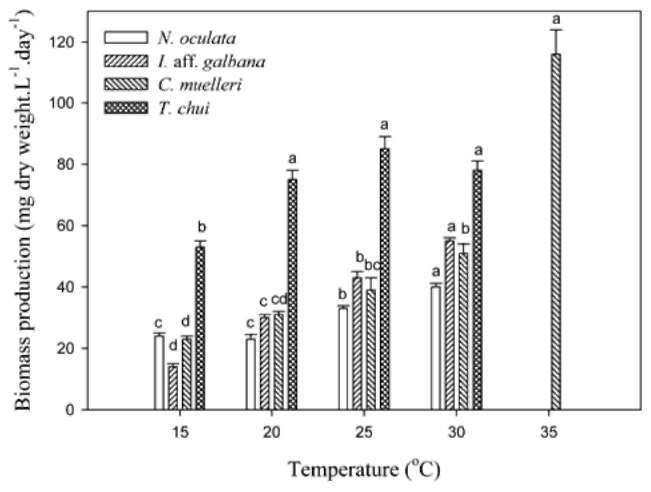
Dry weights per cell of I. aff. galbana and C. muelleri were temperature-independent at temperatures of < 30°C (Table 2). Among these four microalgal species, only C. muelleri could grow at 35°C. The dry weight of C. muel-leri at 35°C was much higher than those at < 30°C. The dry weight of N. oculata at 20, and 25°C was significantly lower than those at 15, and 30°C. However, the dry weight of T. chui at 30°C was significantly higher than those at < 25°C.
|
||
|
Temperature effect on the growth rates (p) and biomass production
|
||
|
Microalgae in log-phase were inoculated into ASW only with
13 mg-L-1 TAN-N and 4 mg-L-1 PO43-- P at 25°C. The 3-day microalgal culture growth rates are given in Figure 1. First-day growth rates for N. oculata and C. mu-elleri were significantly higher than those for the second and third days. First-day growth rates of I. aff. galbana and T. chui were comparable to those for the second day but significantly higher than those for the third day. There-fore, first-day growth rates for the four microalgal species could be used to evaluate their growth rates and their TAN uptake rates at different temperatures.
|
||
|
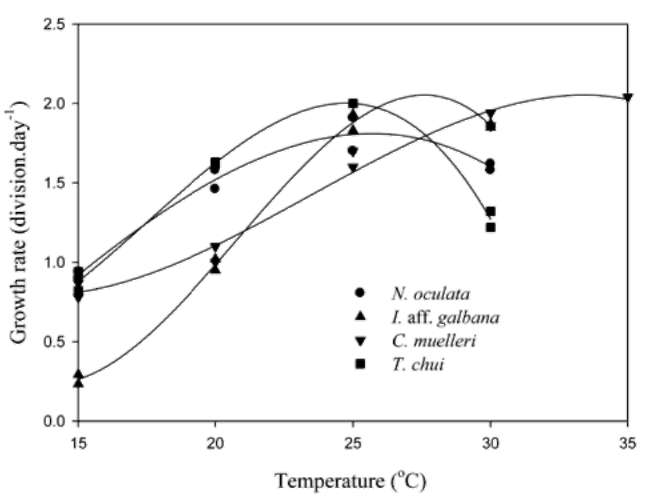
Figure 2 shows the microalgal growth rates at different temperature levels. The f values for N. oculata and T. chui were significantly greater than those for I. aff. galbana and C. muelleri at 20°C, while values of the latter were significantly greater than the former at 30°C. Based on polynomial regression data, respective optimal temperatures for N. oculata, I. aff. galbana, C. muelleri, and T. chui growth were 26, 28, 33, and 25°C.
|
||