|
|
|
|
|
|
|
|
|
|
|
|
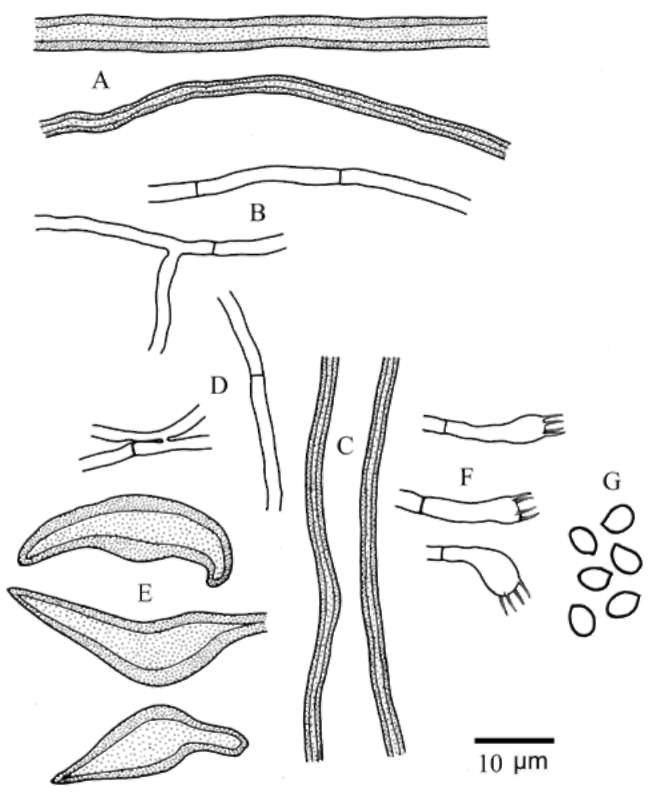
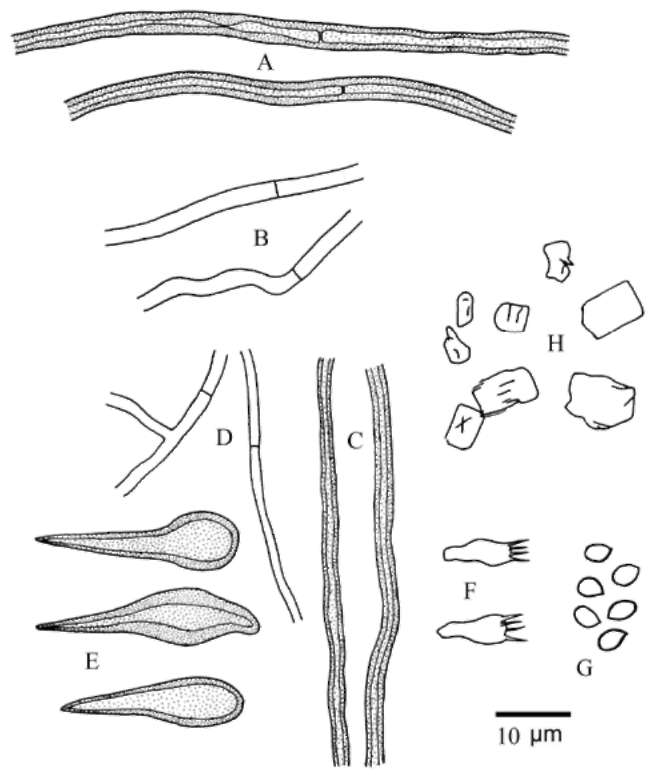
2-3 fm diam., thick-walled. Hymenial setae variably abundant, thick-walled, brown or dark-brown, ventricose or subulate, 18-35 x 7-14 fm. Basidia subclavate, 11-16 x 4-5 fm, 4-sterigmate. Basidiospores broadly ellipsoid, yellowish, brownish yellow, or brownish, smooth, slightly thick-walled or thick-walled, (3.8-)4.0-4.9(-5.1) x (3.0-) 3.1-3.9(-4.1) μm (Wu 0903-1: L = 4.27 ± 0.16 μm, W =
3.36 ± 0.19 μm, Q = 1.27 (n = 30); FS656176: L = 4.41 ± 0.30 μm, W = 3.58 ± 0.23 μm, Q = 1.23 (n = 30); CA: L = 4.25 ± 0.20 μm, W = 3.41 ± 0.14, Q = 1.25 (n = 30); TFM F-21771: L = 4.31 ± 0.20 μm, W = 3.33 ± 0.20, Q = 1.29 (n = 30); TH: L = 4.37 ± 0.28 μm, W = 3.36 ± 0.22 μm, Q = 1.30 (n = 30)), IKI-, CB-.
|
|
|
|
Additional specimens examined. CHINA. Jilin Prov., Baishan City, on Morus sp., 7 Jun 2009, FS656176 (TNM F24926), FS656177 (TNM F24927), FS656178 (TNM F24928). Hunan Prov., Shuanfeng Co., on Morus sp.,OCT 2006, BZ-A (TNM F24794), BZ-C (TNM F24795).
Jiangxi Prov., Nanchang, on Morus sp., 2004, purchased
basidiocarp, CA (TNM F24796), CB (TNM F24797), CC
(TNM F24798). JAPAN. Miyazaki Pref., Aya, Kawanaka,
on Morus sp., 3 Oct 2003 (TFM F21475, voucher of the
culture WD-2261), Suki-mura, on M. bombycis (= M.
australis), 2-3 Aug 1938 (TFM F1324, ex TNS F206786).
Tokyo Pref., Hachiyojima Is., 18 Aug 2005 (TFM F21771,
voucher of the culture WD-2300), Hachijo Is., on Morus
sp., 22 Apr 1950 (TFM F1995, possibly the voucher of the
culture WD-1222), Mikura Is., Apr 2002 (TFM F20053),
Toshima Is., on M. alba, 16 May 1951 (TFM F23284).
Tottori Pref., Kawahara-cho, on Morus sp., 8 Jul 1972
(TFM F23285). Ishikawa Pref., Tsurugi-cho, on Morus
sp., 3 Aug 1983 (TFM F20059). Gumma Pref., Kiryu, on
Morus sp., 6 Dec 2004 (TFM F21303). Hyogo Pref., Sayocho,
on Morus sp., 15 Jul 1961 (TFM F23282). TAIWAN.
Nantou Co., Hsingyi Hsiang, Hoshe, on Morus sp., 2005,
purchased basidiocarp, TH (TNM F24799), TJ (TNM
F24800), TM (TNM F24801), TN (TNM F24802). Taitung
Co., Yienping Hsiang, on Morus sp., May 2011, purchased
basidiocarp, Wu 1105-4 (TNM F24925).
|
|
|
|
Distribution. China, Japan, Korea (Lim et al., 2003), Taiwan.
|
|
|
|
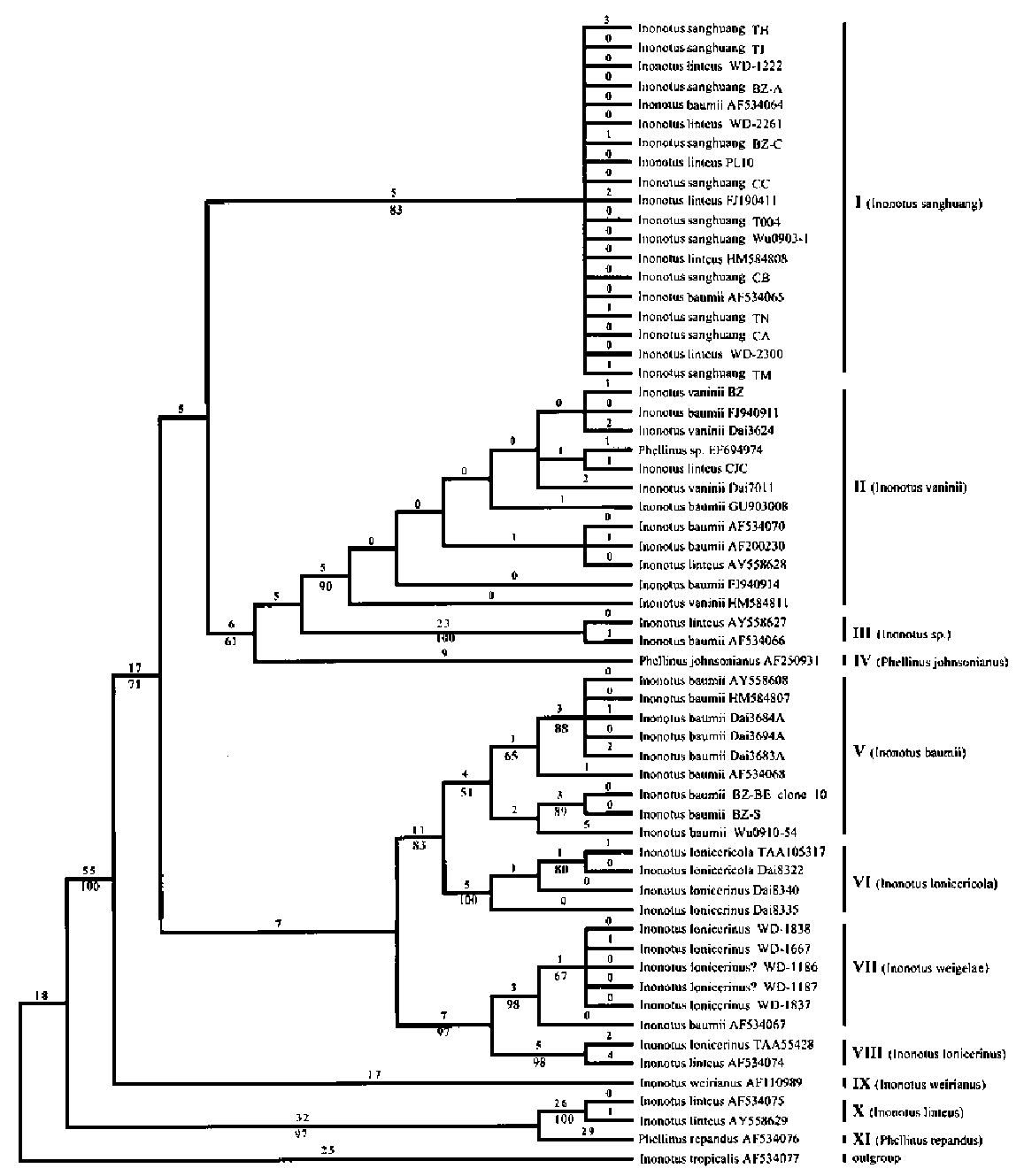
Remarks. Inonotus sanghuang is a new species, proposed in this paper to represent the real sanhuang mushroom, known in China for more than 1000 years. This species was previously wrongly assigned to I. linteus or I. baumii, mainly due to their similar morphological characteristics. The basidiocarp of I. sanghuang fairly resembles those of I. baumii and I. vaninii. The young hymenial surface of I. sanghuang is golden-yellow, becoming brownish yellow to yellowish brown with age. The hymenial surface of I. baumii is pale-brown or brown. The adaxial part of the applanate form of the I. sanghuang basidiocarp is generally flat, slightly convex or slightly concave, and occasionally tuberculate, while that of I. baumii is more or less concave. Inonotus sanghuang only grows on Morus, while I. baumii mainly grows on Syringa, and occasionally on other angiosperms. Inonotus vaninii shares a golden-
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
yellow hymenial surface with I. sanghuang, especially in young basidiocarps. Inonotus vaninii may display re-supinate or pileate basidiocarps, depending on different growth orientations, while I. sanghuang generally lacks the resupinate form. The pileus surface of I. vaninii is moderately sulcate (< 3 furrows/cm), and bears a wide yellow marginal zone. The pileus surface of I. sanghuang is densely sulcate (> 3 furrows/cm), and the yellow marginal zone of its pileus is usually distinct when young; this zone grows much narrower or disappears with age. Moreover, I. vaninii only grows on Populus.
|
|
|
|
Inonotus linteus was commonly used for naming sanghuang mushroom, but is distributed in tropical America and Africa, not in E Asia, does not grow on Morus, and has subglobose and larger basidiospores (4.35.5 x 3.8-4.8 im, according to Dai and Xu (1998)).
|
|
|
|
Inonotm vaninii (Ljub.) T. Wagner & M. Fisch., Mycolo-
gia 94: 1009 (2002).
|
|
|
|
≡Phellinus vaninii Ljub., Botanicheskie Materialy 15: 115
(1962).
|
|
|
|
Specimens examined. CHINA. Jilin Prov., Antu Co., Changbaishan Forest Reserve, on living tree of Populus davidiana, 28 Jul 1993, Dai 812 (IFP, TNM F6269); on living tree of P. davidiana, 8 Sep 1995, Dai 1978 (IFP, TNM F6272); on fallen trunk of P. davidiana, 8 Sep 1995, Dai 1980 (IFP, TNM F6273); on living tree of P. davidi-ana, 14 Sep 1995, Dai 2102 (IFP, TNM F6275); on fallen trunk of P. davidiana, 2005, Dai 7011 (IFP); Yanbian Autonomous State, Helung Co., on P. davidiana, 3 Aug 2010, FS656175 (TNM F24803). Locality in China unknown, on Populus, BZ-2031 (TNM F24804). RUSSIA. Reservatum Bolshechechtshirski apud urbem Chabarovsk, pr. ostium fluminis Tshirka, on P. davidiana, 24 Aug 1982 (TAA 105094, TNM F15789). Commercially cultivated immature basidiome with no collection information, CJC01 (TNM F24805).
|
|
|
|
Distribution. North America, North China, Russian Far East, Japan (Nunez and Ryvarden, 2000), Korea (this study).
|
|
|
|
Remarks. Dai (2010) offered a comprehensive morphological description for I. vaninii. This species grows exclusively on Populus (Nunez and Ryvarden, 2000; Dai, 2010). Resupinate or pileate basidiocarps can be present in I. vaninii according to different growth orientations. The resupinate part develops on the underside below the woody substratum while the pileate part is formed from the lateral side of the woody substratum. Many strains of I. vaninii were previously assigned to I. baumii or I. linteus (Table 1) according to GenBank information, implying that I. vaninii was often regarded as conspecific with the real sanghuang (I. sanghuang), mainly due to their shared golden-yellow hymenial surfaces. Dai (2010) reported that the golden and lustrous pileus marginal zone of I. vaninii becomes bloody red with KOH. A similar color reaction also occurs in I. sanghuang.
|
|
|
|
|
|
|
|