|
|
|
|
|
|
|
|
|
|
|
|
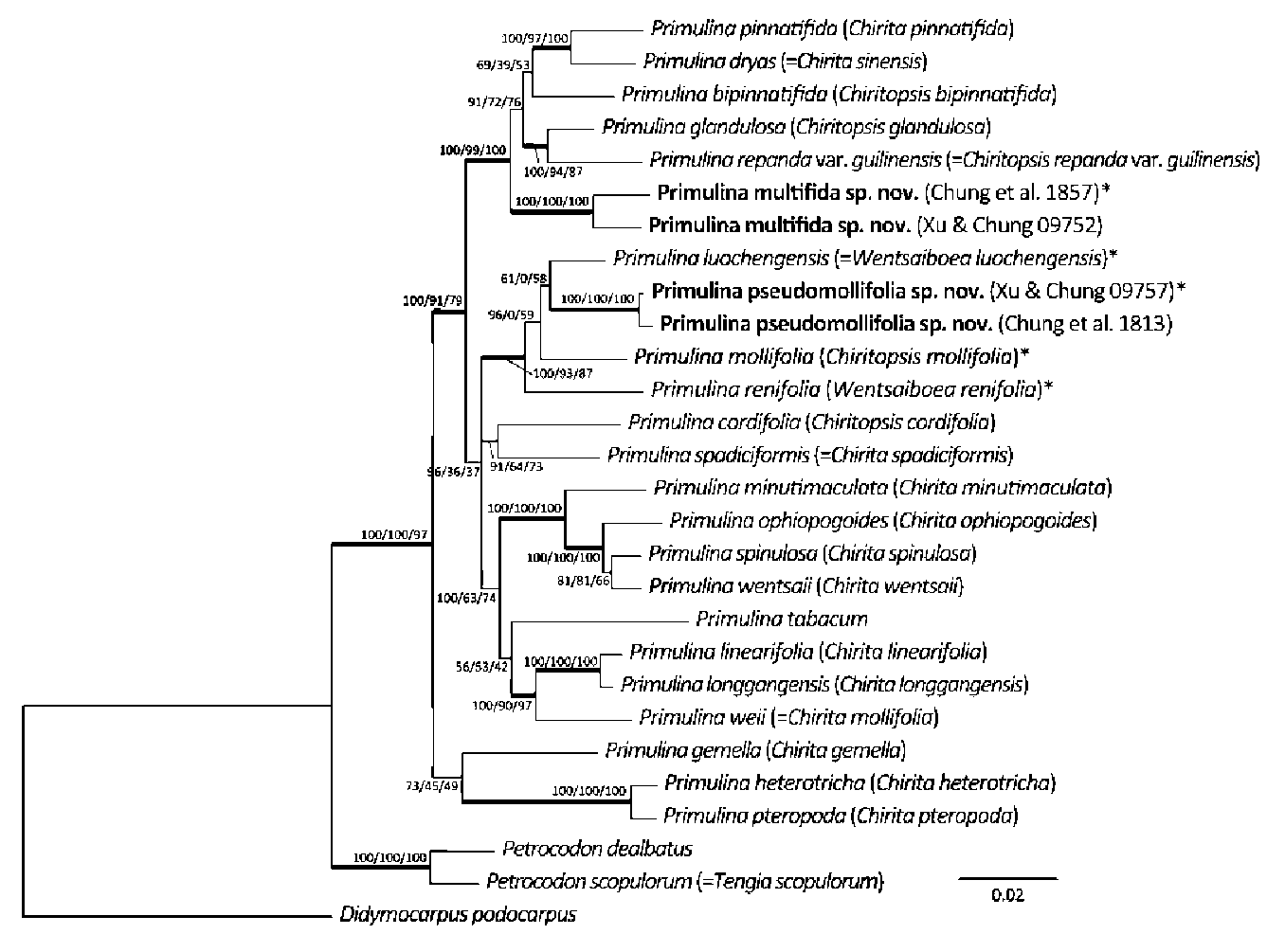
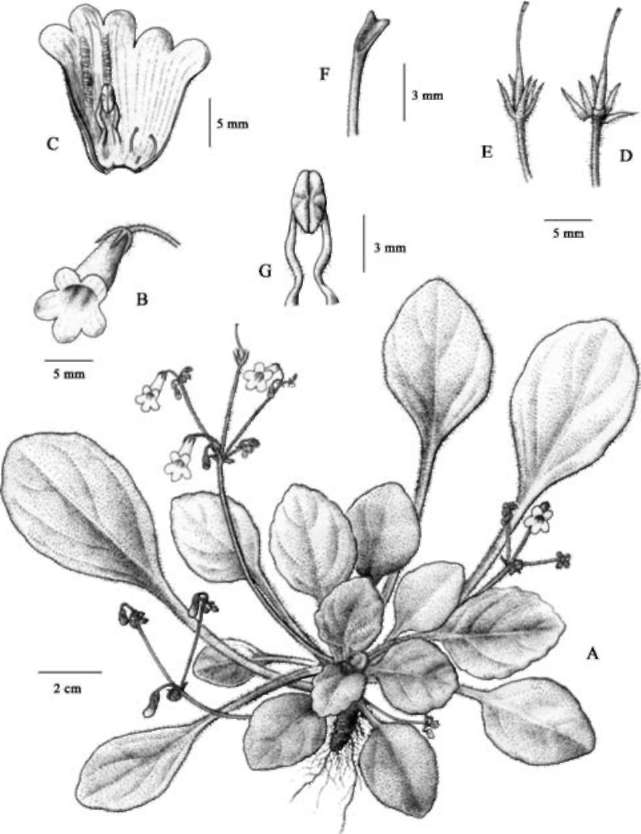
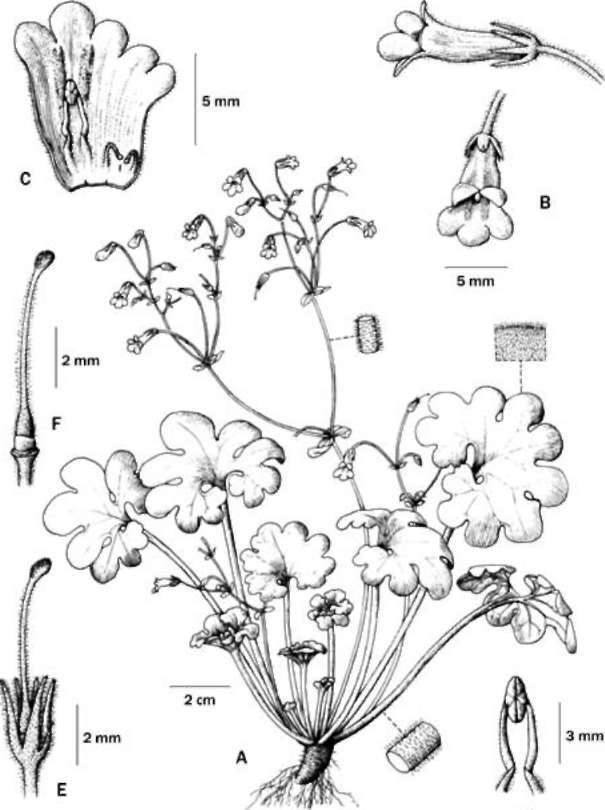
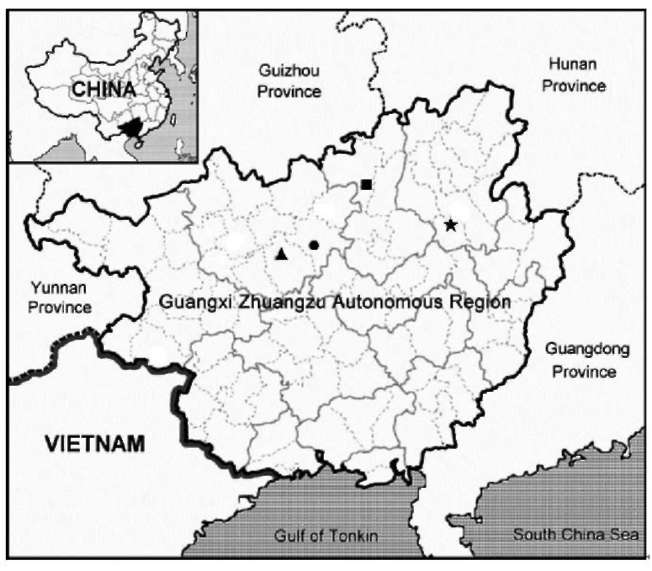
Based on DNA sequences of nuclear ITS and a chloroplast trnL-F intron spacer, we confirmed the generic placement of the two new Gesneriad species, adding two distinct taxa to the splendidly diverse Primulina. Primulina multifida and P. pseudomollifolia are localized to two limestone caves in Guangxi, as are most plant species confined to limestone caves along the Sino-Vietnam-ese border. However, these habitats are very fragile, highly vulnerable to ever increasing regional economic activities (Clements et al., 2006). Remarkably, in the past decade, botanists exploring the limestone ecosystems on the Sino-Vietnamese border have uncovered numerous new species from diverse groups ranging from ferns to conifers to various lineages of angiosperms (e.g., Farjon et al.,
2002; Peng et al., 2008; Wu et al., 2009; Yu et al., 2009; Lin et al., 2010; Jiang et al., 2011; Pan et al., 2011). The sheer number of these recent discoveries highlights the extraordinary plant diversity in the region, and the urgency of a thorough inventory and conservation assessment of these precious biodiversity hotspots.
|
|
|
|
Acknowledgments. The authors are grateful to Prof. Fa-Nan Wei (IBK) for the Latin diagnosis and Miss Tze-Lin Pan and Hsuan Chang for collecting DNA sequence data. We also thank Mr. Shun-Qing He (IBK) and Mr. Yun-Xi Zhu (IBK) for the handsome illustrations. We appreciate the helpful comments of the anonymous reviewers. This study was supported by the National Natural Science Foundation of China (Grant no. 41161011) and the Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences to Yan Liu (IBK), and National Geographic Society Grant # 835807 to Ching-I Peng (HAST), Yan Liu (IBK) and Kuo-Fang Chung (NTUF).
|
|
|
|
|
|
|
|
Clements, R., N.S. Sodhi, M. Schilthuizen, and P.K.L. Ng. 2006. Limestone karsts of southeast Asia: Imperiled arks of biodi-versity. BioScience 56: 733-742.
|
|
|
|
Doyle, J.J. and J.L. Doyle. 1987. A rapid DNA isolation procedure for small quantitites of fresh leaf tissue. Phytochem. Bull. 19: 11-15.
|
|
|
|
Fang, D. and D.H. Qin. 2004. Wentsaiboea D. Fang et D.H. Qin, a new genus of Gesneriaceae from Guangxi, China. Acta Phytotax. Sin. 42: 533-536.
|
|
|
|
Farjon, A., N.T. Hiep, D.K. Harder, P.K. Loc, and L. Averyanov. 2002. A new genus and species in Cupressaceae (Conifer-ales) from northern Vietnam, Xanthocyparis vietnamensis. Novon 12: 179-189.
|
|
|
|
He, H. and L.B. Zhang. 2011. Polystichum cavernicola, sp. nov. (sect. Haplopolystichum, Dryopteridaceae) from a karst cave in Guizhou, China and its phylogenetic affinities. Bot. Stud. 52: 121-127.
|
|
|
|
|
|
|
|
|
|
|
|
|
Hou, M.F., Y. Liu, Y. Kono, and C.-I Peng. 2009. Aspidistra dax-inensis (Ruscaceae), a new species from limestone areas in Guangxi, China. Bot. Stud. 50: 371-378.
|
|
|
|
Hou, M.F., J. Lopez-Pujol, H.N. Qin, L.S. Wang, and Y. Liu. 2010. Distribution pattern and conservation priorities for vascular plants in southern China: Guangxi Province as a case study. Bot. Stud. 51: 377-386.
|
|
|
|
Lin, C.R., C.-I Peng, Y. Kono, and Y. Liu, 2010. Aspidistra obconica, Asparagaceae [Ruscaceae], a new species from limestone areas in Guangxi, China. Bot. Stud. 51: 263-268.
|
|
|
|
Liu, Y, Y. Kono, C.R. Lin, W.B. Xu, and C.-I Peng. 2011. Aspidistra erecta (Asparagaceae), a new species from limestone areas in Guangxi, China. Bot. Stud. 52: 367-373.
|
|
|
|
Jiang, R.H., X.C. Zhang, and Y. Liu. 2011. Asplenium cornutis-simum (Aspleniaceae), a new species from karst caves in Guangxi, China. Brittonia 63: 83-86.
|
|
|
|
Ku, S.M., Y. Kono, and Y. Liu. 2008. Begonia pengii (sect. Coe-locentrum, Begoniaceae), a new species from limestone areas in Guangxi, China. Bot. Stud. 49: 167-175.
|
|
|
|
Li, J.M. and Y.Z. Wang. 2007. Phylogenetic reconstruction among species of Chiritopsis and Chirita sect. Gibbosaccus (Gesneriaceae) based on nrDNA ITS and cpDNA trnL-F sequences. Syst. Bot. 32: 888-898.
|
|
|
|
Li, Z.Y. and Y.Z. Wang. 2004. Plants of Gesneriaceae in China. Henan Science and Technology Publishing House, Zheng-zhou.
|
|
|
|
Lin, C.R., C.-I Peng, Y. Kono, and Y. Liu. 2010. Aspidistra
obconica, Asparagaceae [Ruscaceae], a new species from limestone areas in Guangxi, China. Bot. Stud. 51: 263-268.
|
|
|
|
Liu, Y., W.B. Xu, and B. Pan. 2010. Wentsaiboea tiandengensis sp. nov. and W. luochengensis sp. nov. (Gesneriaceae) from Karst caves in Guangxi, southern China. Nordic J. Bot. 28: 739-745.
|
|
|
|
Lopez-Pujol, J., F.M. Zhang, H.Q. Sun, T.S. Ying, and S. Ge.
2011. Centres of plant endemism in China: places for survival or for speciation? J. Biogeogr. 38: 1267-1280.
|
|
|
|
Moller, M., A. Forrest, Y.G. Wei, and A. Weber. 2011. A molecular phylogenetic assessment of the advanced Asiatic and Malesian didymocarpoid Gesneriaceae with focus on non-monophyletic and monotypic genera. Pl. Syst. Evol. 292: 223-248.
|
|
|
|
Moller, M., M. Pfosser, C.G. Jang, V. Mayer, A. Clark, M.L. Hollingsworth, M.H. J. Barfuss, Y.Z. Wang, M. Kiehn, and A. Weber. 2009. A preliminary phylogeny of the 'Didymo-carpoid Gesneriaceae' based on three molecular data sets: incongruence with available tribal classifications. Amer. J. Bot. 96: 989-1010.
|
|
|
|
|
|
|
|
Pan, B., W.H. Wu, D.X. Nong, and W.B. Xu. 2010. Chiritopsis
longzhouensis, a new species of Gesneriaceae from limestone areas in Guangxi, China. Taiwania 55: 370-372.
|
|
|
|
Peng, C.-I, S.M. Ku, Y. Kono, K.F. Chung, and Y. Liu. 2008.
Two new species of Begonia (sect. Coelocentrum, Begoni-
|
|
|
|
|
|
|
|