HUANG — Leaf trypsin inhibitor and its peptides with antioxidant activities
217
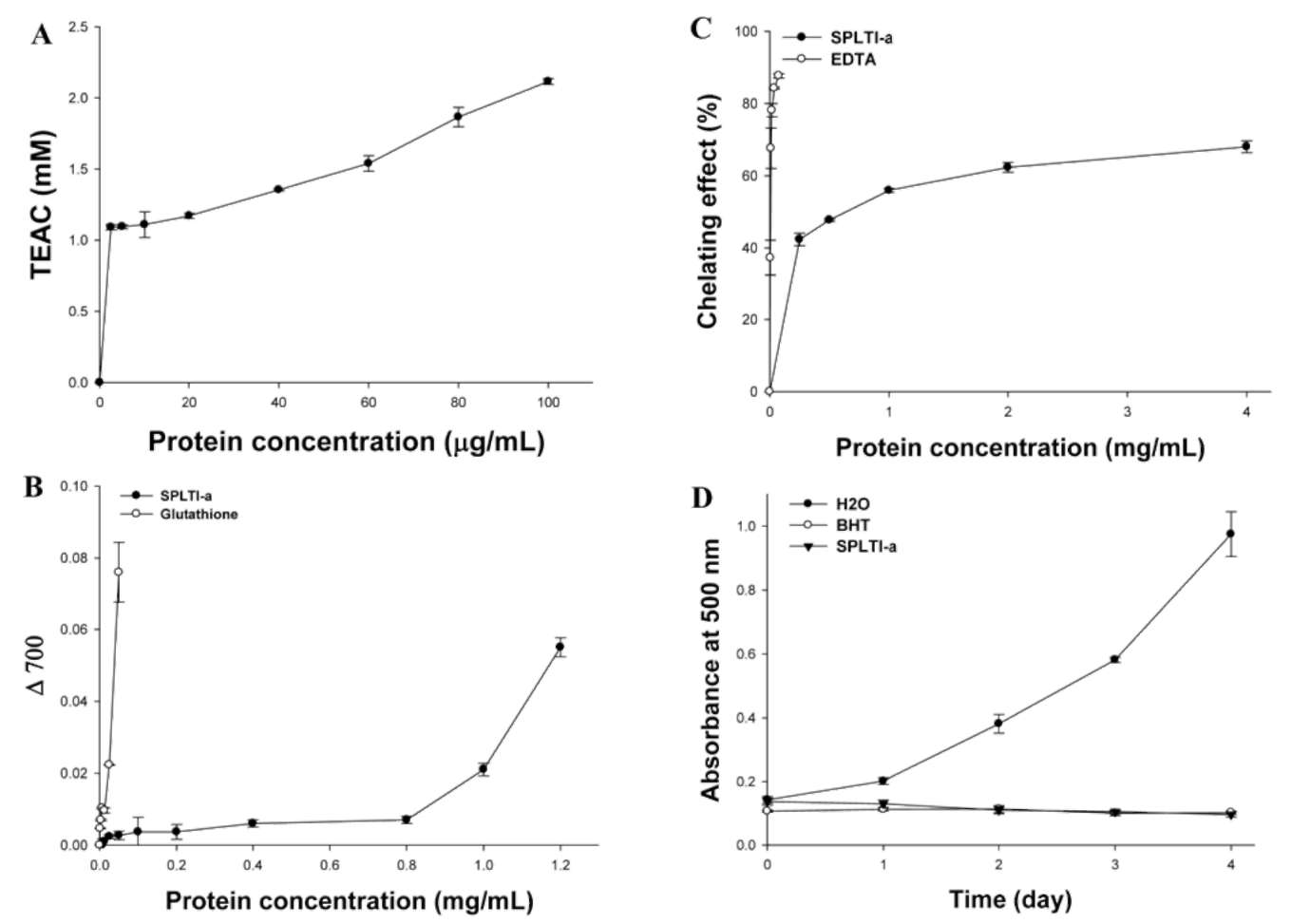
Determination of antioxidant activity by the ferric thiocyanate (FTC) method
each fraction contained 2 mL of which the absorbance at 280 nm was then determined.
The FTC method was adapted from the method of Osawa and Namiki (1981). Twenty mg/mL of samples dissolved in 4 ml of 99.5% (w/v) ethanol were mixed with linoleic acid (2.51%, v/v) in 99.5% (w/v) ethanol (4.1 mL), 0.05 M phosphate buffer pH 7.0 (8 mL) and deionized water (3.9 mL) and kept in a screw-cap container at 40°C in the dark. Then, to 0.1 mL of this solution was added 9.7 mL of 75% (v/v) ethanol and 0.1 mL of 30% (w/v) ammo-nium thiocyanate. Precisely 3 min after the addition of 0.1 mL of 20 mM ferrous chloride in 3.5% (v/v) hydrochloric acid to the reaction mixture, the absorbance at 500 nm of the resulting red color [Fe (SCN)2+, Fe3+ was formed after linoleic acid peroxide was produced and Fenton reaction occurred.] was measured every 24 h until the day when the absorbance of the control reached the maximum value. The inhibition of linoleic acid peroxidation was calculated as (%) inhibition = 100 - [(absorbance increase of the sam-ple/absorbance increase of the control) x 100]. All tests were run in duplicate and analyses of all samples were run in triplicate and averaged.
Statistical analysis
Means of triplicates were calculated. Student's t test was used for comparison between two treatments. All data (expressed as percent of control value) were means± SE. A difference was considered to be statistically significant whenp < 0.05, p < 0.01 or p < 0.001.
RESULTS and DISCUSSION
Purification of expressed SPLTI-a
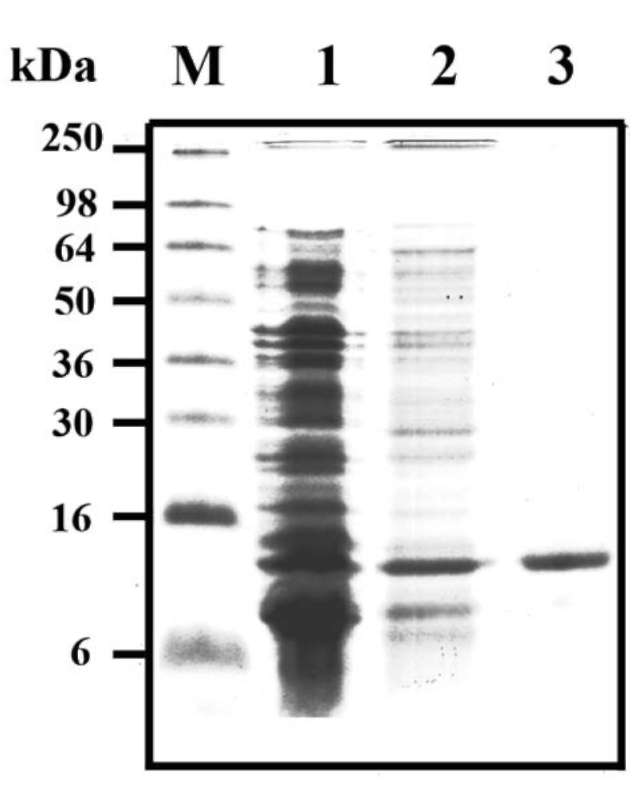
SPLTI-a cDNA clones from sweet potato leaf was isolated. SPLTI-a was subcloned in a pQE-32 expression vector in E. coli and SPLTI-a was produced with a 6x His-tag at the N-terminus. SDS-PAGE analysis of crude extracts from transformed E. coli (M15) showed a high level of a polypeptide with the expected molecular mass (ca. 8 kDa). This polypeptide was found as a soluble protein in the supernatant (Figure 1, lane 2), and was absent in protein extracts obtained from E. coli transformed with
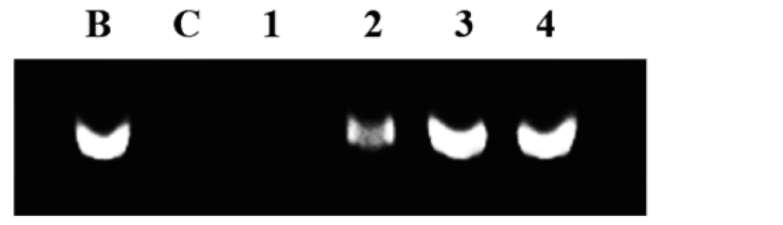
Protection of SPLTI-a against hydroxyl radical-induced calf thymus DNA damage
The hydroxyl radical was generated by Fenton reaction according to the method of Kohno et al. (1991). The 15 μL reaction mixture containing SPLTI-a (1.25, 2.5, 5, and 10 mg/mL), 5 μL of calf thymus DNA (1 mg/mL), 18 mM FeSO4, and 60 mM hydrogen peroxide were incubated at room temperature for 15 min. Then 2μL of 1 mM EDTA was added to stop the reaction. Blank test contained only calf thymus DNA and the control test contained all reaction components except SPLTI-a. The treated DNA solutions were subjected to agarose electrophoresis and then stained with ethidium bromide and examined under UV light.
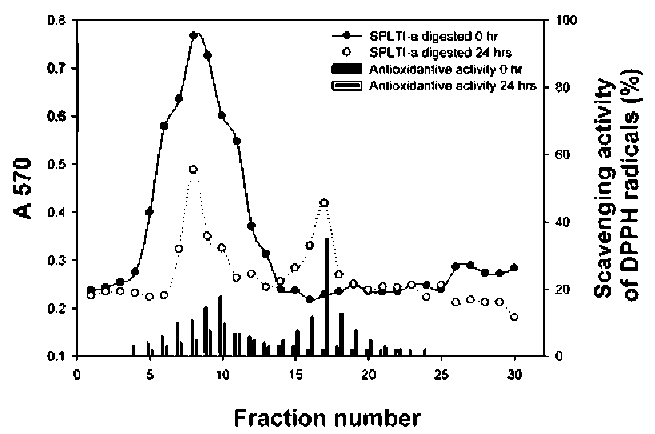
Determination of the antioxidative activity of SPLTI-a tryptic hydrolysates
Six mg of SPLTI-a was dissolved in 1 mL of 0.1 M KCl buffer (pH 8.0). Then 0.1 mL (12 mg) of trypsin was add-ed at 37°C for 0 and 24 h. After hydrolysis, 0.5 mL of 0.5 M Tris-HCl buffer (pH 8.3) was added, and the solution was heated at 100°C for 5 min to stop enzyme reaction. The trypsin was heated before SPLTI-a hydrolysis for the 0 h reaction. Each of the 60 μL SPLTI-a hydrolysates was used for determinations of the DPPH antioxidative activi-ties by spectrophotometry (Mine et al., 2004; Qian et al., 2008).
Figure 1. SDS-PAGE analysis of purified recombinant sweet potato leaf trypsin inhibitor (SPLTI-a). Crude extracts from E. coli (M15) transformed with pQE30 (lane 1) or with pQE30-SPLTI-a (lane 2) were analyzed by 15% (w/v) SDS/PAGE with 10 μg protein applied on each lane, and then the gel was stained with Coomassie blue G-250. Molecular masses of standard pro-teins are indicated at the left of the figure. His-tagged SPLTI-a was purified by Ni2+-chelated affinity chromatography (lane 3). The experiments were done twice and a representative one is shown.
Chromatograms of tryptic hydrolysates of SPLTI-a on a Sephadex G-50 column
The unhydrolyzed SPLTI-a and tryptic SPLTI-a hydro-lysates at 24 h were separated by Sephadex G-50 chroma-tography (1 x 60 cm). The column was eluted with 20 mM Tris-HCl buffer (pH 7.9). The flow rate was 30 mL/h, and