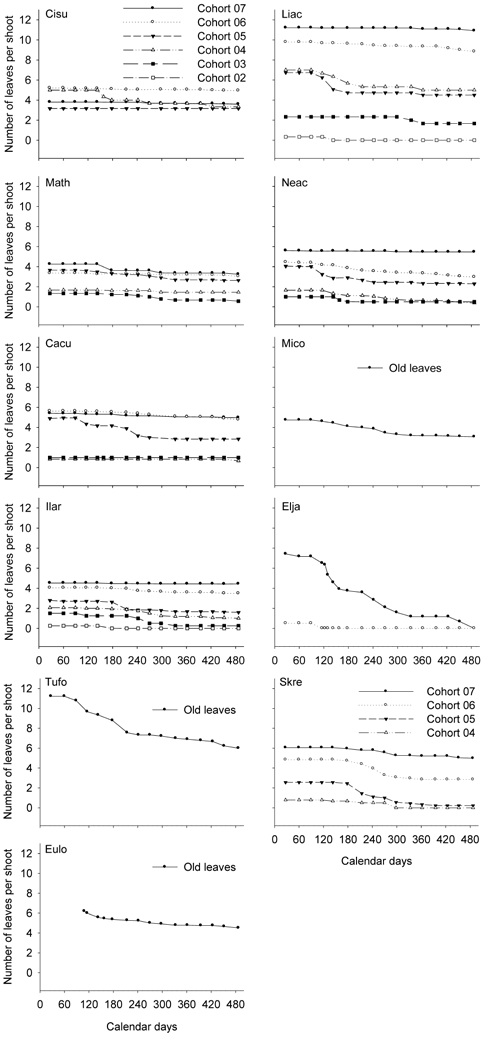
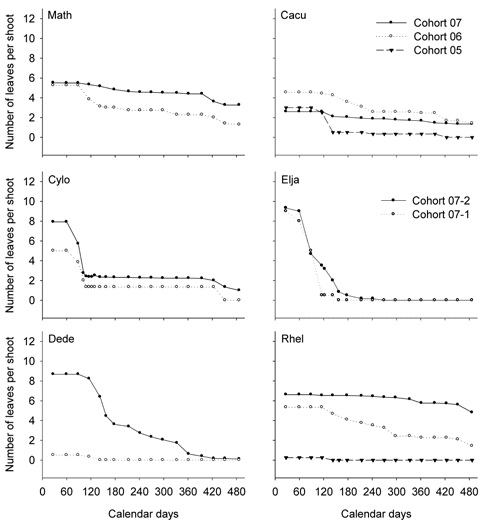
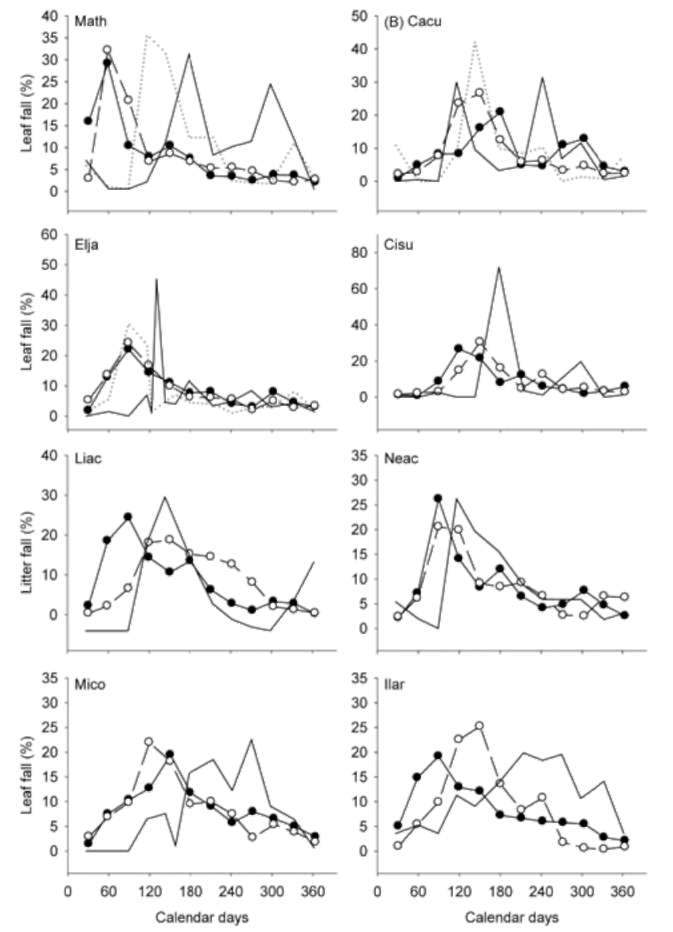
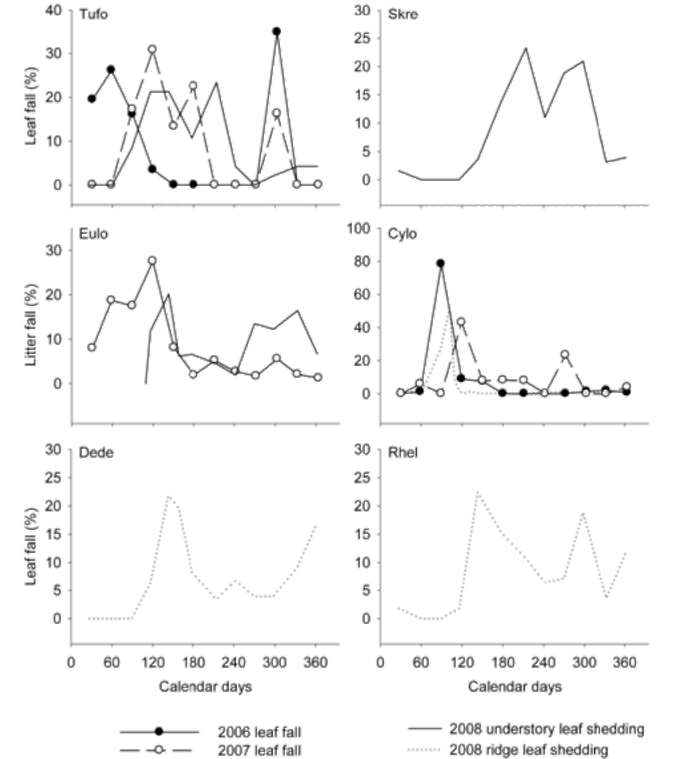
LU et al. ― Hardwood leaf lifespan
259
of the survey (Figures 3 and 4). After 15 months of the survey, this single leaf had fallen from all the twigs in 3 species, L. cubeba, E. japonicas, and D. dentiger, but strangely persisted in the other species.
Similarly, the curves depicting the leaf shedding in the cohorts of 2007 are rather flat in many species, indicating that these cohorts rarely shed leaves (Figures 3 and 4). However, 1 or 2 leaves fell from the cohorts produced before 2007.
Compared to the other species, C. longinux, E. japoni-cas, and D. dentiger had more distinctive patterns of leaf shedding (Figures 3 and 4). For C. longinux, the cohorts of both 2006 and 2007 shed leaves heavily in March and April. For E. japonicas and D. dentiger, the cohort of 2007 continued to shed leaves until all leaves were gone.
In M.formosana, T. formosana and E. loquaiana, 3 species with the succeeding types of leaf emergence, the old leaves shed slowly and continuously all year round (Figure 3).
Overall patterns of leaf shedding
In Figure 5, we plotted the temporal patterns of the leaf shedding of both the newly emerged and old leaves,
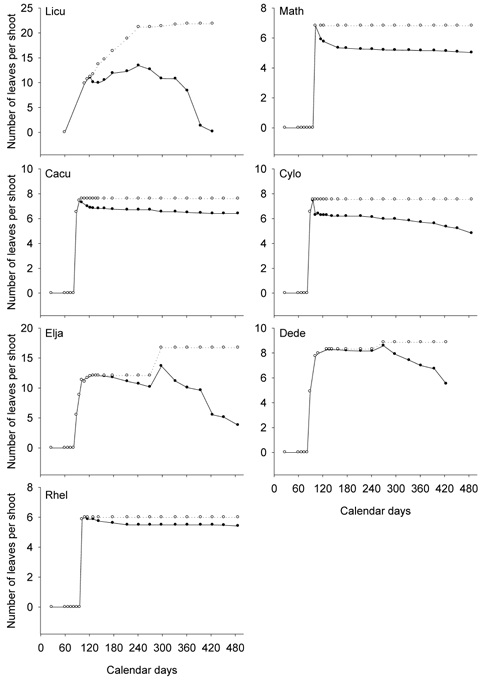
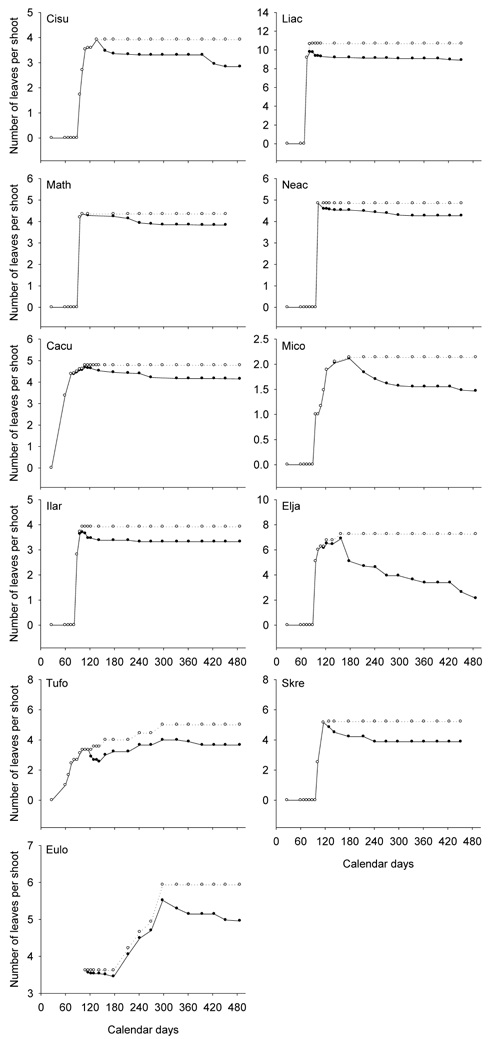
Figure 1. Leaf production and shedding of the understory species. The cumulative numbers of the newly emerged leaves on the current shoots are shown by open circles (o), and the numbers of the survived leaves on the current shoots by filled circles (•). Each point represents the average of 2 to 3 individuals. Jan. 1, 2008 was "1" on the axis of Calendar days. The last observation was on May 2, 2009, "487" on the axis of Calendar days. The complete scientific names of the abbreviations are listed in Table 1.
Figure 2. Leaf production and shedding of the ridge species. The cumulative numbers of the newly emerged leaves on the current shoots are shown by open circles (o), and the numbers of the survived leaves on the current shoots by filled circles (•). Each point represents the average of 2 to 3 individuals. Jan. 1, 2008 was "1" on the axis of Calendar days. The last observation was on May 2, 2009, "487" on the axis of Calendar days. The complete scientific names of the abbreviations are listed in Table
1.