302
Botanical Studies, Vol. 53, 2012
with 10 mL deionized water), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), ferrous sulfate, L-glutamic acid y-monohydroxamate (GAH, G2253), horseradish peroxi-dase (148 units/mg solid), and semicarbazide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Hydrogen peroxide (33%) was from Wako Pure Chemical Industry (Osaka, Japan). Other chemicals and reagents were from Sigma Chemical Co. (St. Louis, MO, USA).
Determination of SSAO inhibitory activity
SSAO inhibitory activities were determined by spectro-photometric method according to Szutowicz et al. (1984) with modifications (Liu et al., 2011a,b) using hydrogen peroxide to plot the SSAO standard curve. The total 200 jaL reaction solution (containing 50 joL of 200 mM phosphate buffer, pH 7.4, 50 joL of 8 mM benzylamme, SSAO, and different amounts of AAH or GAH, and semicarba-zide) was placed at 37°C for one hour and then heated at 100°C boiling water for 5 min to stop reaction. After cooling and brief centrifugation, the 90 j L reaction solution was isolated and added to 710 j L of spectrophotometric solution containing 200 j L of 200 mM phosphate buffer (pH 7.4), 100 joL of 2 mM ABTS solution, and 25 joL of horseradish peroxidase (10 j g/mL). Means of triplicates were measured. Deionized water was used instead of AAH or GAH or semicarbazide as a blank experiment. One unit of SSAO activity was defined as amounts of enzyme to produce one nanomole of hydrogen peroxide during one hour under above-mentioned reaction condition. The changes of absorbance at 420 nm were recorded during 1 min and expressed as AA420nm/min. The relative SSAO activity (%) was calculated with the equation:
(ΔA420nm/min,blank- ΔA420nm/min,sample)÷ΔA420nm/min,blank*100%
(ΔA420nm/min,blank- ΔA420nm/min,sample)÷ΔA420nm/min,blank*100%
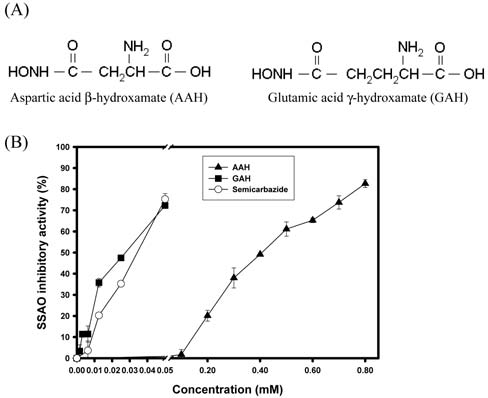
Figure 1. The effects of AAH and GAH on SSAO from bovine plasma. (A) The structure of AAH and GAH, and (B) inhibition of SSAO activities by AAH, GAH, and semicarbazide, respectively. The SSAO inhibition (%) was calculated with the equation: (ΔA420 nm/minblank- ΔA420 nm/minsample)÷ΔA420 nm/minblank*100%
isolated from leaves of Acacia confusa (Lee et al., 2006) and geraniin isolated from Phyllanthus urinaria (Lin et al., 2008) showed dose-dependent inhibitory activities against SSAO. The hydroxamic acid derivative of hydroxyurea showed MAO and SSAO inhibitory activities (Liu et al., 2010). The synthesized alginic acid hydroxamate (Liu et al., 2007), synthesized galacturonic acid hydroxamate (Liu et al., 2011a) and glucuronic acid hydroxamate (Liu et al., 2011b) were reported to have SSAO inhibitory activities. The extracts of Taiwanofungus camphoratus (Chang-Chih) showed dose-dependent inhibitory activities against SSAO (Wang et al., 2007).
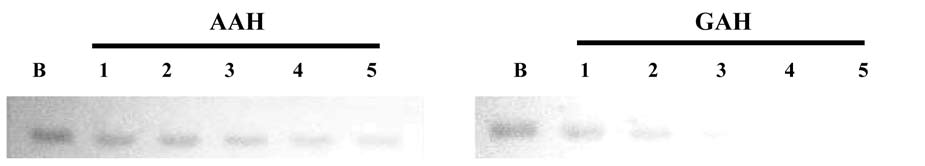
Plasma SSAO activity stains on 10% native polyacrylamide gels
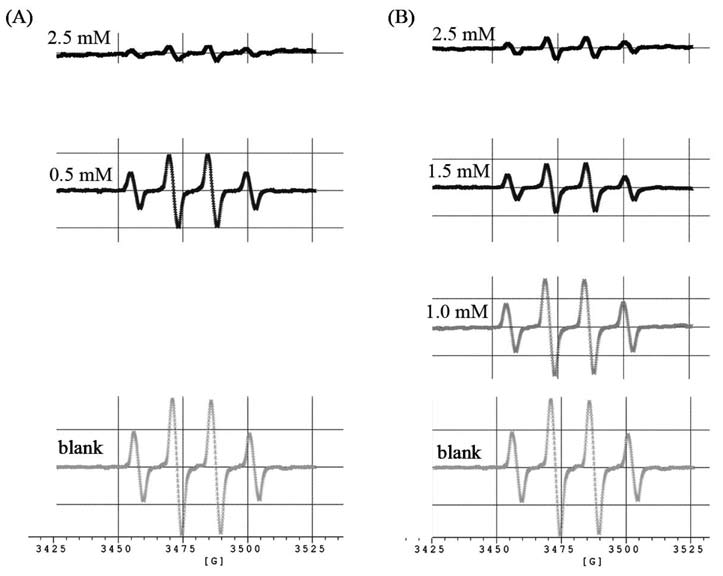
Our previous report showed that aspar-tic acid p-hydroxamate (AAH) and glutamic acid y-monohydroxamate (GAH) (Figure 1A) exhibited angio-tensin converting enzyme inhibitory activities, and scavenging activities against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and superoxide radicals (Liu et al., 2004). The AAH exhibited antitumor activity on L5178Y leukemia (Thomasset et al., 1991), therapeutic effect on friend eryth-roleukemia (Tournaire et al., 1994a) and antiproliferative activity on friend virus-infected erythropoietic progenitor cells (Tournaire et al., 1994b). In this research, AAH and GAH were effectively to inhibit SSAO activities from bovine plasma in a dose-dependent manners, and also showed effectively scavenging activities against hydroxyl radicals.
The SSAO activity staining on a 10% polyacrylam-ide gel was according to the method of Lee et al. (2002). Bovine plasma (23.45 units) was premixed with AAH or GAH (0, 1.5, 1.75, 2, 2.25, and 2.5 mM) overnight and prepared for electrophoresis. When native PAGE was finished, the gels were balanced for 20 min twice in 50 mM Tris-HCl buffer (pH 7.9) before activity staining. The process of plasma SSAO activity staining was as below. Eighty mg benzylamine and 40 mg AEC was dissolved in 10 mL dimethylformamide and then added to 40 mL, 50 mM Tris-HCl buffer (pH 7.9) as the substrate solution, in which the gel was submerged and shaked for 5 min. Then, 200 j L horseradish peroxidase (5 mg/mL) was added. The gel was gentle shaking at room temperature. The gel was destained with 10% acetic acid and then washed with distilled water.
MATERIALS AND METHODS
Materials
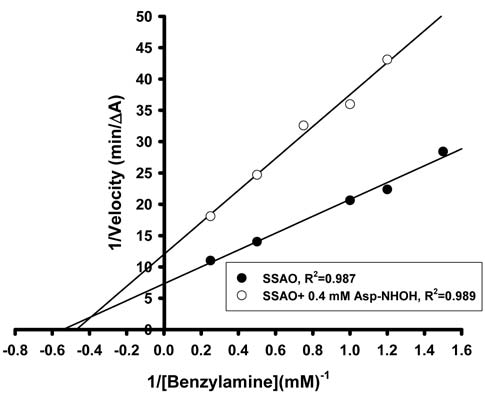
Determination of the kinetic constant of bovine SSAO in the presence of AAH
L-Aspartic acid p-hydroxamate (AAH, A6508), ben-zylamine, 2, 2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, ABTS), bovine plasma (P-4639, reconstitute
The Km and Vmax of bovine SSAO (18.76 units) and the