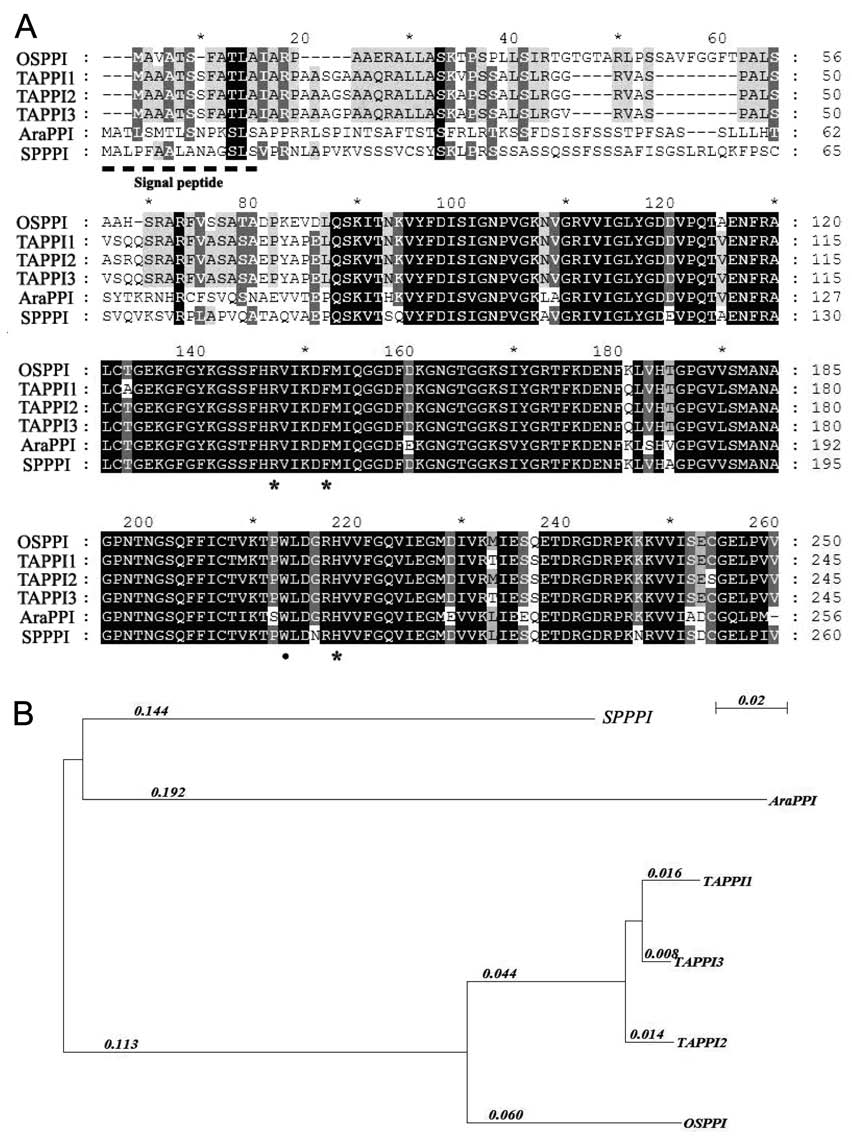
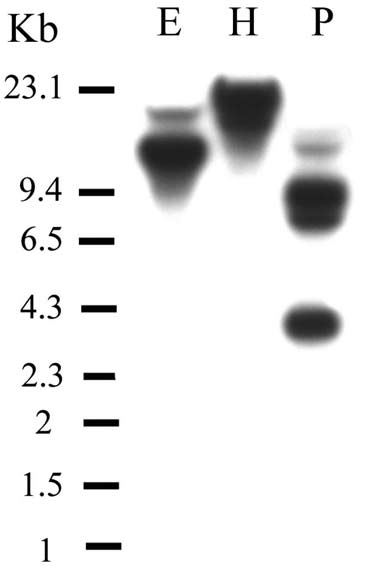
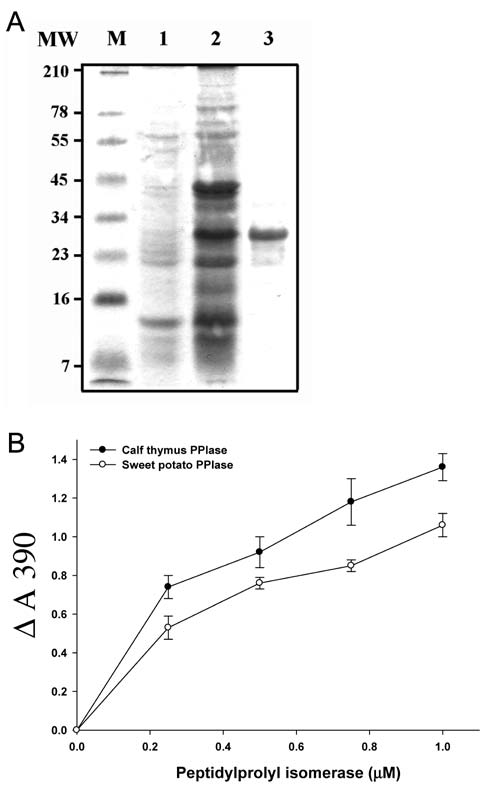
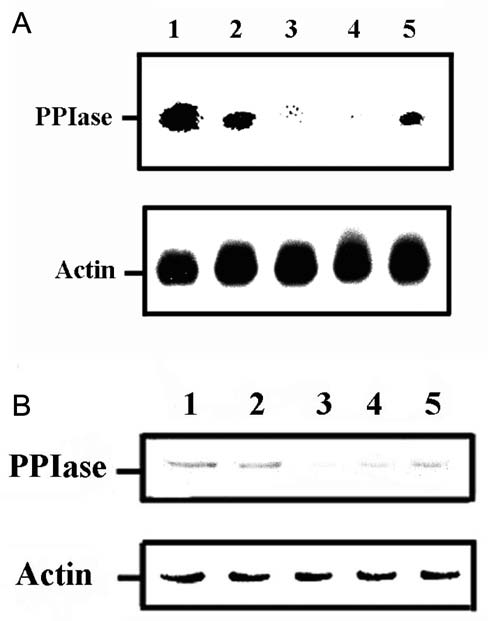
chem. Biophys. 371: 149-162.
Handschumacher, R.E., M.W. Harding, J. Rice, R.J. Drugge, and D.W. Speicher. 1984. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226: 544-547.
Huang, D.J., H.J. Chen, W.C. Hou, C.D. Lin, and Y.H. Lin.
2004. Active recombinant Thioredoxin h protein with anti-oxidant activities from sweet potato (Ipomoea batatas [L.] Lam. 'Tainong 57') storage roots. J. Agric. Food. Chem. 52:
4720-4724.
Huang, D.J., H.J. Chen, W.C. Hou, and Y.H. Lin. 2005. Expression and localization of protein disulfide isomerase cDNA from sweet potato (Ipomoea batatas [L.] Lam. 'Tainong 57') storage roots. Plant Sci. 169: 776-784.
Huang, D.J., H.J. Chen, W.C. Hou, and Y.H. Lin. 2006. Sweet
potato (Ipomoea batatas [L.] Lam 'Tainong 57') storage roots mucilage with antioxidant activities in vitro. Food
Chem. 98: 774-781. Huang, G.J., M.J. Sheu, H.J. Chen, Y.S. Chang, and Y.H. Lin.
2007a. Inhibition of reactive nitrogen species in Vitro and ex Vivo by trypsin inhibitor from sweet potato 'Tainong 57' storage roots. J. Agric. Food. Chem. 55: 6000-6006.
Huang, D.J. H.J. Chen, W.C. Hou, and Y.H. Lin. 2007b. Sweet potato (Ipomoea batatas [L.] Lam. 'Tainong 57') storage roots mucilage with antioxidant activities in vitro. Food
Chem. 98: 774-781. Huang, G.J. H.J. Chen, Y.S. Chang, M.J. Sheu, and Y.H. Lin.
2007c. Recombinant sporamin and its synthesized peptides with antioxidant activities in vitro. Bot. Stud. 48: 133-140.
Huang, G.J., H.C. Lai, Y.S. Chang, T.L. Lu, M.J. Sheu, H.Y.
Chang, and Y.H. Lin. 2008a. Antimicrobial, dehydroascor-bate reductase and monodehydroascorbate reductase activities of defensin from sweet potato (Ipomoea batatas [L.] Lam. 'Tainong 57') storage roots. J. Agric. Food. Chem. 56:2989-2995.
Huang, S S., G.J. Huang, Y.L. Ho, Y.H. Lin, H.J. Hung, T.N.
Chang, M.J. Chan, J.J. Chen, and Y.S. Chang.' 2008b. An-tioxidant and antiproliferative activities of the four Hydro-cotyle species from Taiwan. Bot. Stud. 49: 311-322.
Horowitz, D.S., E.J. Lee, S.A. Mabon, and T. Misteli. 2002. A
cyclophilin functions in pre-mRNA splicing. EMBO J. 21:
470-480.
Kern, G., D. Kern, F.X. Schimid, and G. Fischer. 1994. Reassessment of the putative chaperone function of prolyl cis/trans-isomerase. FEBS Lett. 348: 145-148.
Krzywicka, A., J. Beisson, A.M. Keller, J. Cohen, M. Jerka-Dziadosz, and C. Klotz. 2001. KIN241: a gene involved in cell morphogenesis in Paramecium tetraurelia reveals a novel protein family of cyclophilin-RNA interacting proteins (CRIPs) conserved from fission yeast to man. Mol. Microbiol. 42: 257-267.
Laxa, M., J.K. Onig, K.J. Dietz, and A. Kandlbinder. 2007. Role
of the cysteine residues in Arabidopsis thaliana cyclophilin CYP20-3 in peptidyl-prolyl cis-trans isomerase and redox-related functions. Biochem. J. 401: 287-297.