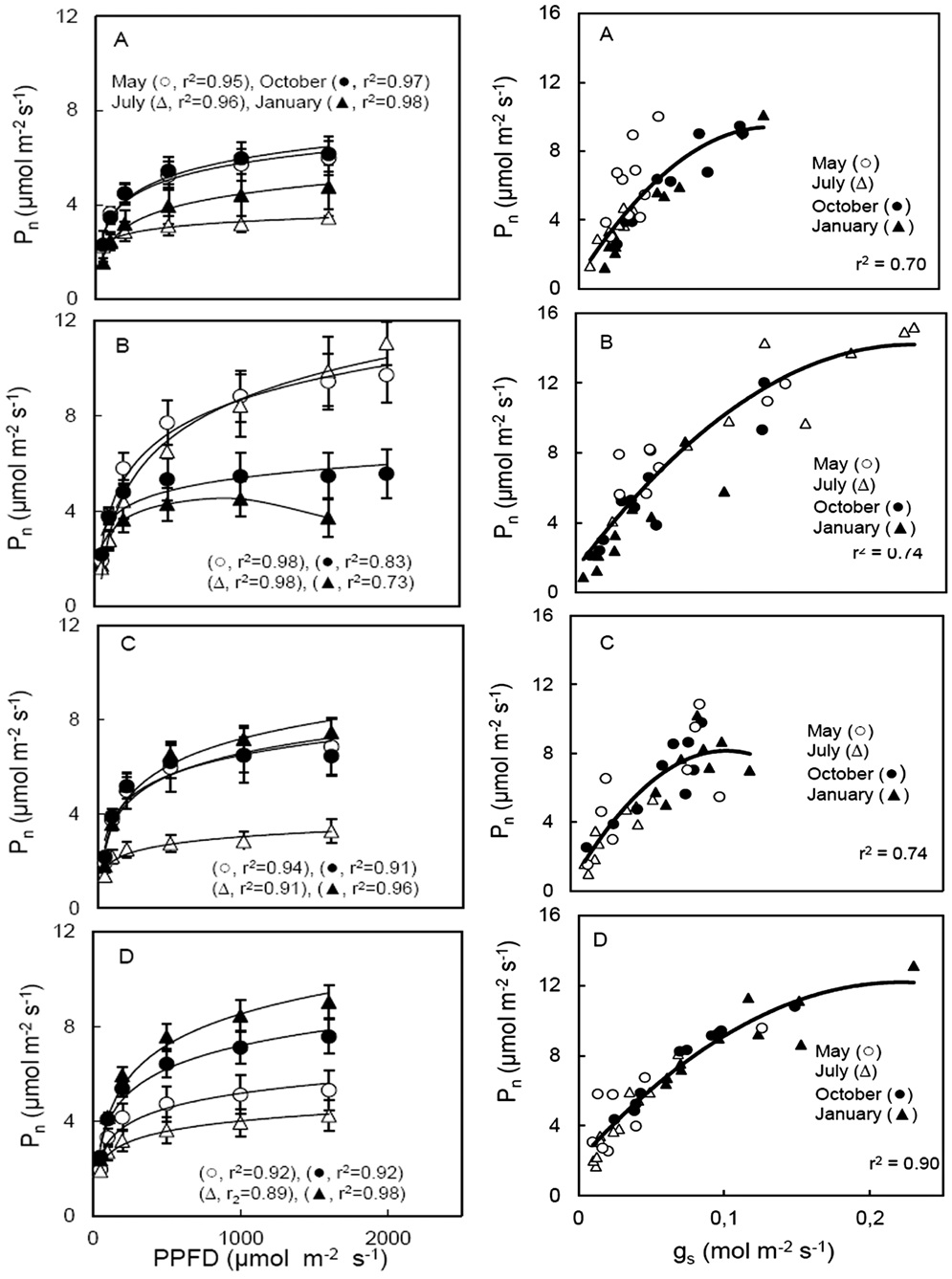
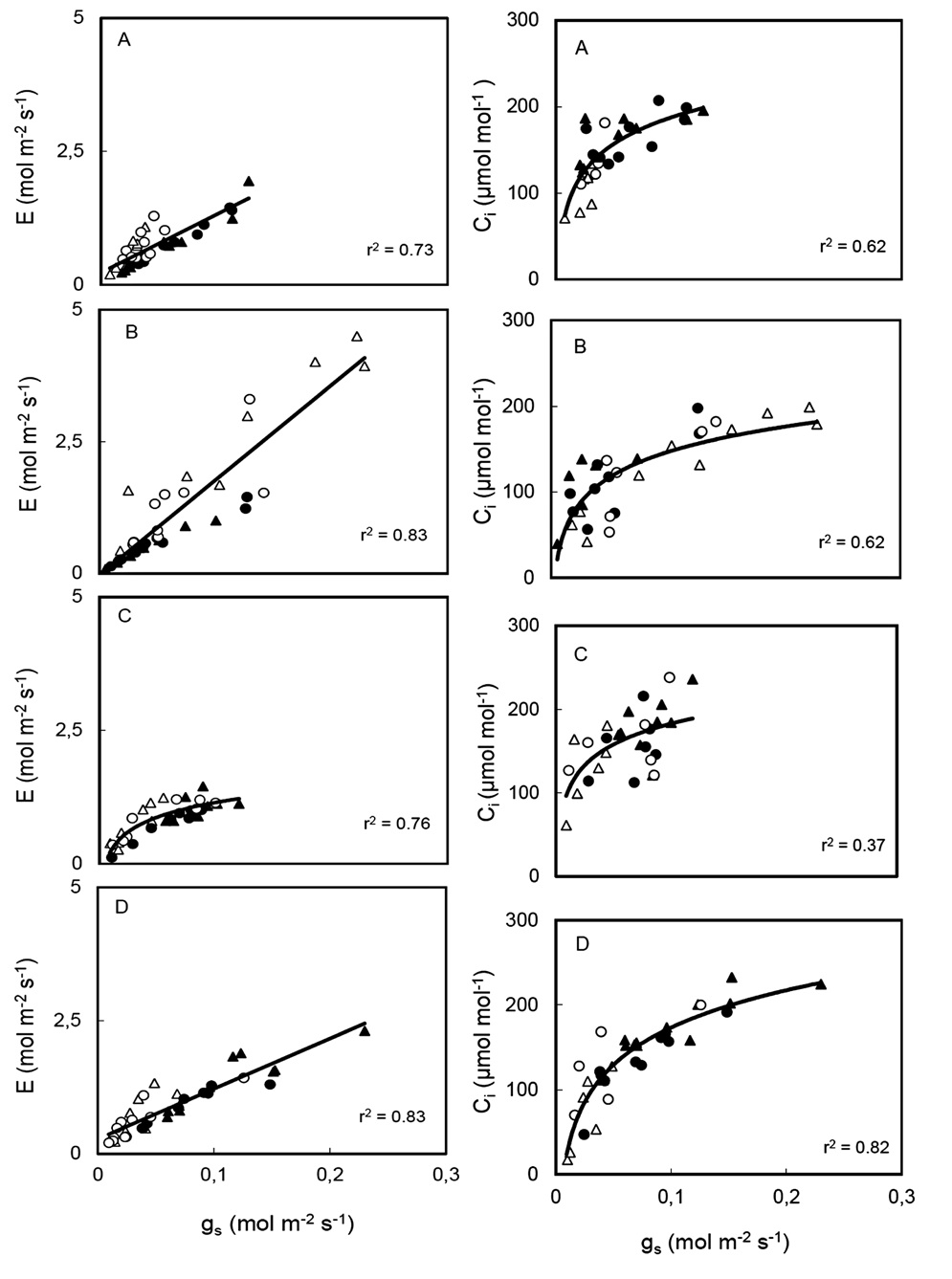
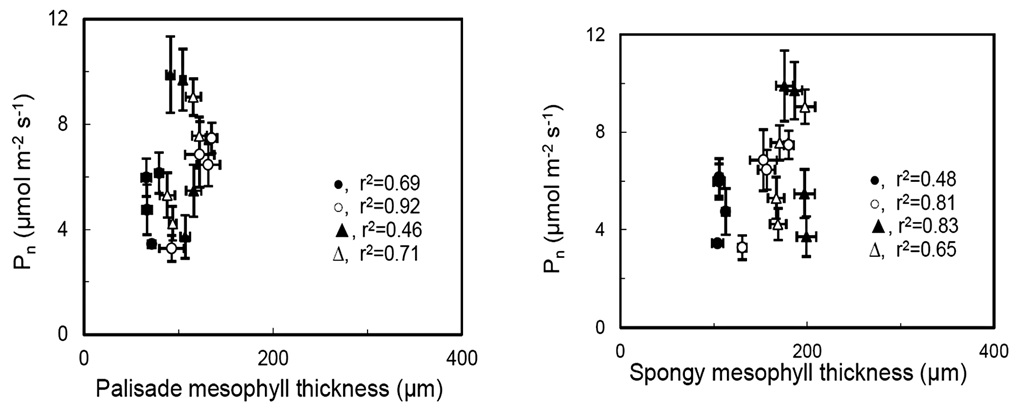
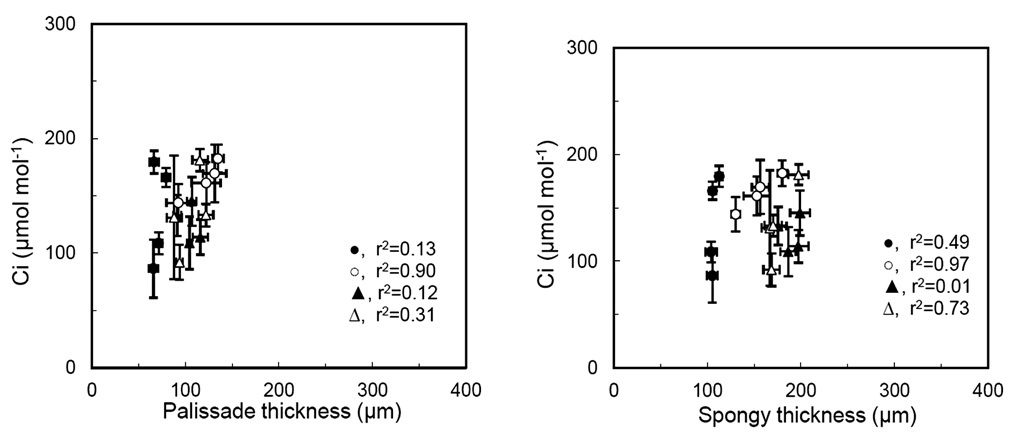
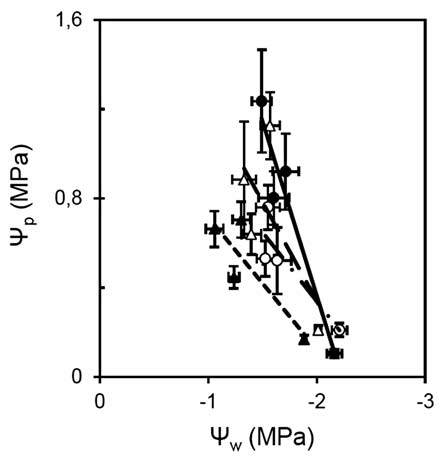
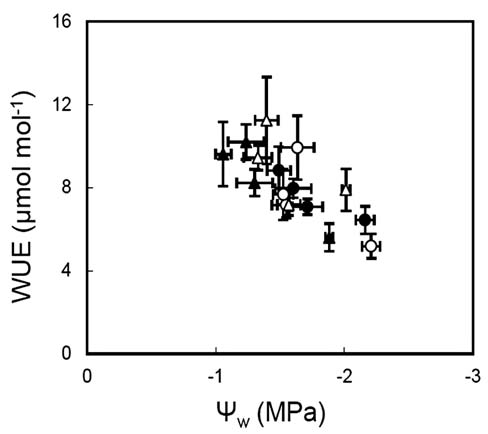
Arena, C., L. Vitale, and V de Santo. 2008. Photosynthesis and photoprotective strategies in Laurus nobilis L. and Quercus ilex L. under summer drought and winter cold. Plant Bio-syst. 142: 472-479.
Badger, M.R., O. Bjorkman, and P.A. Armond. 1982. An analysis of photosynthetic response and adaptation to temperature in higher plants: temperature acclimation in the desert evergreen Nerium oleander L . Plant Cell Environ. 5: 85-99.
Barker, D.H. 1995. Vegetation and Slopes: Stability, Protection and Ecology. Thomas Telford, London, 296 pp.
Beerling, D.J. and P.J. Franks. 2010. The hidden cost of transpiration. Nature 464: 495-496.
Chaves, M.M., J.S. Pereira, J. Marco, M.L. Rodrigues, C.P. Ricardo, I. Osorio, T. Faria, and C. Pinheiro. 2002. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 89: 907-916.
Christodoulakis, N.S. and K.A. Mitrakos. 1987. Structural analysis of sclerophylly in eleven evergreen phanerophytes in Greece. In J.D. Tenhunen, F.M Catarino, O.L Lange and W.C. Oechel (eds.), Plant response to stress. Springer-Verlag, Berlin, pp. 547-551.
Christodoulakis, N.S. 1993. Air pollution effects on the guard cells of the injury resistant leaf of Laurus nobilis L. Bull. Environ. Contam. Tox. 51: 471-478.
Christodoulakis, N.S., P.-N. Lampri, and C. Fasseas. 2009. Structural and cytochemical investigation of the leaf of silverleaf nightshade (Solanum elaeagnifolium), a drought-resistant alien weed of the Greek flora. Aust. J. Bot. 57:432-438.
Delaney, K.J. 2008. Injured and uninjured leaf photosynthetic responses after mechanical injury on Nerium oleander leaves, and Danaus plexippus herbivory on Asclepias curassavica. Plant Ecol. 199: 187-200.
Driscoll, S.P., A. Prins, E. Olmos, K.J. Kunert, and C.H. Foyer. 2006. Specification of adaxial and abaxial stomata, epidermal structure and photosynthesis to CO2 enrichment in maize leaves. J. Exp. Bot. 57: 381-390.
Faria, T., D. Silverio, E. Breia, R. Cabral, A. Abadia, J.S. Perei-ra, and M.M. Chaves. 1998. Differences in the response of carbon assimilation to summer stress (water deficits, high light and temperature) in four Mediterranean tree species. Physiol. Plant. 102: 419-428.
Galmes, J., H. Medrano, and J. Flexas. 2007. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 175: 81-93.
Hanba, Y.T., S.-I. Miyazawa, and I. Terashima. 1999. The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm temperate forests. Funct. Ecol. 13: 632-639.
Harrison, S.P., C.I. Prentice, D. Barboni, J. Ni, and J.-P. Sutra. 2010. Ecophysiological and bioclimatic foundations for a