ZHANG and YIN ― Photosynthetic acclimation of Meconopsis to irradiance
337
fmol mol-1, returned it to 370 fmol mol-1, and then increased it further. During this period, the leaf temperature, PPFD, and vapor pressure deficit were set to 20°C, 1200 [mol m-2s-1, and 1.0 to 1.5 kPa, respectively.
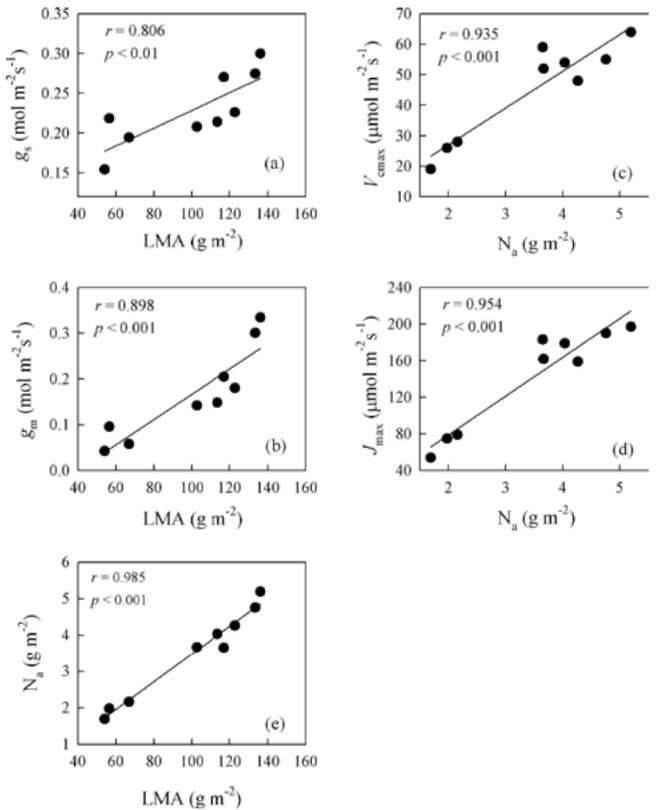
where 1^ was the value of r at 25°C (42.75 [imol mol-1); c and AHa represented a scaling constant of 19.02 and an excitation energy of 37.83 (kJ mol-1), respectively; R was the molar gas constant (8.314 kJ K-1 mol-1); and T was the leaf temperature (°C) (Bernacchi et al., 2001). Values for gm were calculated from our measurements of photosyn-thetic rates at internal CO2 concentrations of 100 to 300 fmol mol-1, with the average value of gm being determined for each leaf (Niinemets et al., 2005). The rate of pho-tosynthetic electron transport (ETR) was obtained from chlorophyll fluorescence measurements.
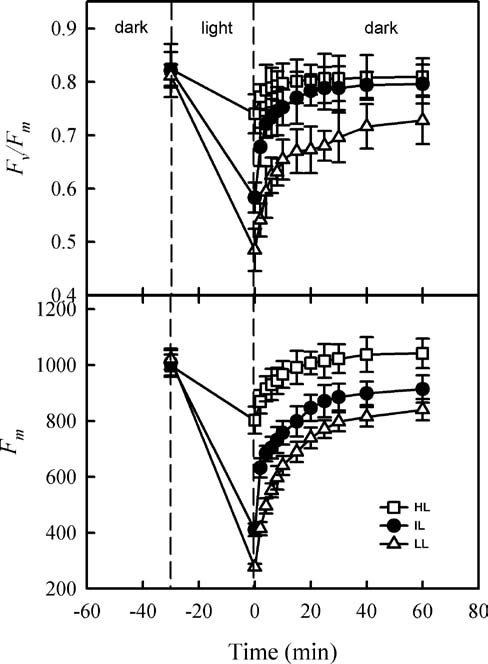
For our recovery experiment, the sample leaves were dark-adapted at 20°C for 30 min before the minimum and maximum fluorescence yields were calculated. They were then illuminated at 2000 fmol m-2s-1 for 30 min. Afterward, the light source was switched off and the samples were allowed to recover in the dark at 20°C. Values for F。 and Fm were recorded after 30 and 60 min of recovery, respectively.
Statistical analysis
Determination of leaf traits
Statistical analysis was performed with SPSS 13.0
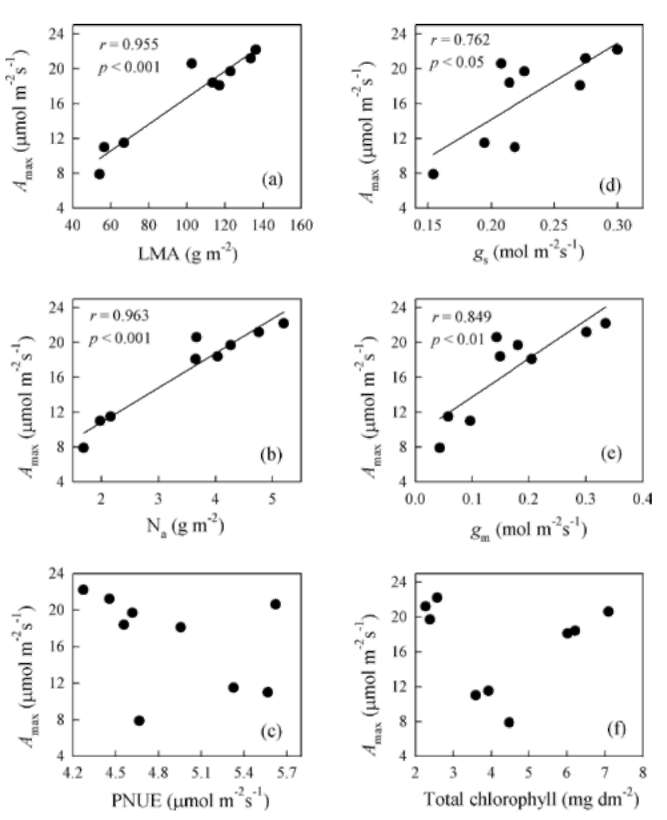
(SPSS Inc., Chicago, IL, USA). To estimate the differences among light treatments, we tested the data for chlorophyll fluorescence, photosynthesis and leaf traits by using one-way ANOVA and LSD multiple comparison tests. The relationships between photosynthetic parameters and leaf traits were assessed through Pearson regression analysis.
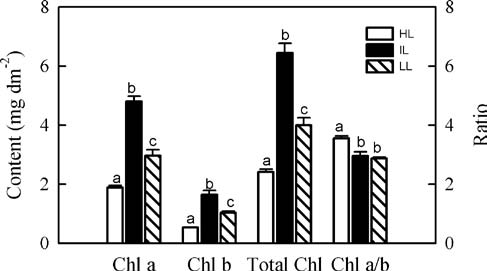
After the measurements of photosynthesis were taken, the leaves were harvested and their areas were determined with a LI-3000A leaf area meter (LI-COR, Lincoln, NE, USA). Dry mass was obtained after the tissues were dried for 48 h at 80°C. From this, LMA was calculated as the leaf dry mass per unit area (g m-2). The leaf N content was assessed by a Leco FP-428 nitrogen analyzer (Leco Cor-poration, St. Joseph, MI, USA). Chlorophyll was extracted per the technique of Moran and Porath (1980), and its con-tent analyzed on a UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan) before being calculated according to the equations of Inskeep and Bloom (1985).
RESULTS
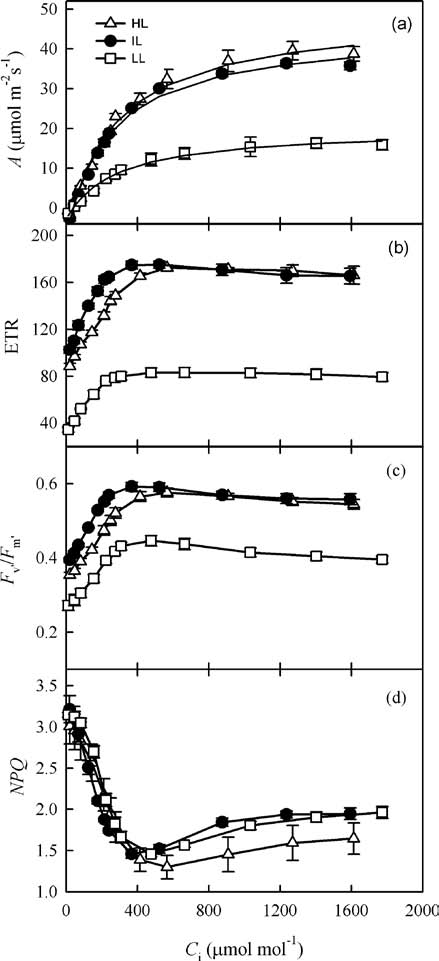
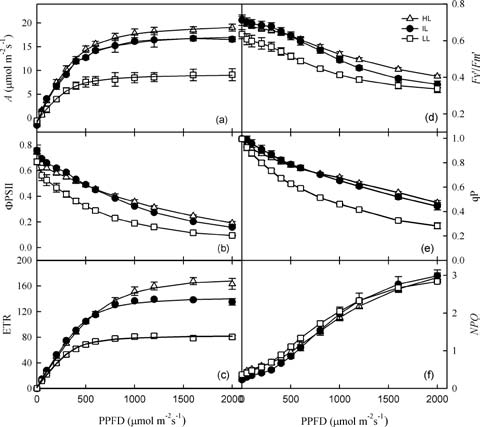
As expected, the photosynthetic rate of M. horridula increased with higher PPFDs (Figure 1). The photosynthetic saturation irradiance was 1174 士 30 [imol m-2s-1 for HL plants, 1158 士 22 [imol m-2s-1 for IL plants, and 972 士 29 [mol m-2s-1 for LL plants. The relationships between ETR and PPFD were similar to those between A and PPFD. Values for F^/Fm, (pPSII, and qP decreased with increas國
Calculation of parameters
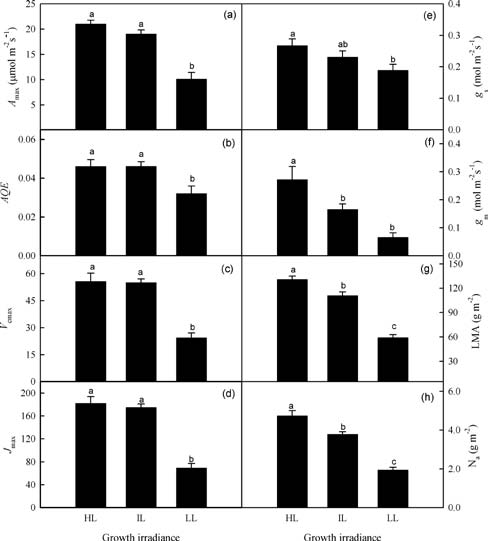
A-PPFD curves were fit by a non-rectangular hyperbola. The light-saturated photosynthetic rate (Amax) and dark respiration (Rd) were determined by using Photosyn Assistant v1.1 (Dundee Scientific, Dundee, Scotland, UK) according to the method of Prioul and Chartier (1977). The photo-synthetic saturation irradiance was defined as the PPFD value at which the photosynthetic rate reached 95% of its maximum (Marschall and Proctor, 2004). Photosynthetic nitrogen-use efficiency (PNUE) was expressed as the ratio of Amax to leaf N content per unit area (Na).
Using A-Ci curves, we calculated the maximum carbox-ylation rate by Rubisco (Vcmax) and light-saturated electron transport (Jmax) with Photosyn Assistant, based on the pho-tosynthetic model of von Caemmerer and Farquhar (1981). Mesophyll diffusion conductance (gm) from the internal air space to the chloroplasts was estimated according to the method of Harley et al. (1992) as:
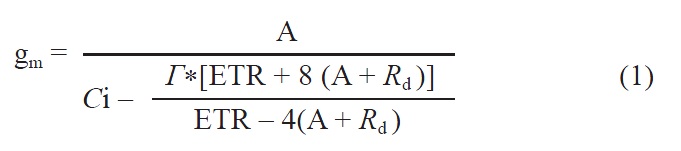
where the rate of dark respiration (Rd) was found from the A-PPFD response curve, and r was the hypothetical CO2 compensation point in the absence of Rd. The value for r* at 20°C was derived from the value at 25°C through the following equation (Bernacchi et al., 2001):
Figure 1. Responses by photosynthetic rate (A), quantum yield of PSII (φPSII), electron transport rate of PSII (ETR), efficiency of excitation energy capture by open reaction centers (Fv,/Fm,), photochemical quenching coefficient (qP), and non-photochemical quenching (NPQ) to photosynthetic photon flux density (PPFD) in Meconopsis horridula grown under different irradiances. Each point represents mean 士 1 SE of 3 measurements from different plants.
Γ*=Γ*25 × exp(c-ΔHa/(R·(T+273.15)))